| Cosmonauts/Astronauts: FISH translocation assay |
Scenario
Cosmic rays are one of the main hazards for long-term manned space missions.
Astronauts/cosmonauts are exposed to various types of high energy charged
particles of the galactic or solar origin. They include electrons, positrons,
protons or heavier ions of high energy. However, the cosmic ray flux around
the space crafts are significantly different from those in free space since
the primary cosmic rays are decelerated by and bombarding the structure
of the space crafts, and hence producing a variety of secondary particles
inside the space crafts. The radiation environment of the astronauts is
thus considerably differs depending on space activity, e.g., missions inside the space craft or extra-vehicle activity. Heavy ion
bombardment may produce one-third of the total neutron flux, and in the
International Space Station (ISS: image by Wikipedia 2015), it has been
estimated that approximately 30 % of the dose received by the astronauts
comes from the albedo neutrons and secondary neutrons created by interactions
in the shielding materials. The neutron energies should extend from thermal
energies up to a few GeV. The total radiation environment is thus completely
different from that we can experience, naturally or experimentally, on
the earth. Moreover, the biological response is also complex, for instance,
like photons, neutrons can activate DNA repair pathways (adaptive response)
as well at low doses while high LET charged particles do not. The health
consequences of hits by HZE particles not clear. The health effects associated
with the manned space exploration in the mixed radiation field and their
quantitative evaluation is the major concern in the current space radiobiology.
Chromosome aberration analysis has been carried out in the cosmonauts/astronauts
to meet this requirement. |
 |
| [1] MIR-18: Analysis by FISH painting, Yang et al. 1997 |
References
Yang, T. C., George, K., Johnson, A. S., Durante, M. and Fedorenko, B.
S. (1997): Biodosimetry results from space flight Mir-18. Radiat. Res.,
S17-S23.
Chromosome
aberration analysis
Blood samples were taken from two crew members involved in a 115-day MIR-18
mission before after flight. Chromosomes were analyzed by FISH painting
technique. Chromosome aberrations significantly increased in post-flight
samples as compared to samples drawn prior to flight. SCE levels were also
studied, but they were not influenced by the flight. Pre-flight samples
were also tested for the dose-response to gamma-irradiation in vitro. The data on these crews are included in the next file (George et al.
2001) as preliminary data.
|
Crew |
Blood |
Cells |
Total aberrations |
Reciprocal translocations |
|
|
member |
sampling* |
scored |
Number |
Frequency (±SD×10-3) |
Number |
Frequency (±SD×10-3) |
|
|
1 |
BF (L-14) |
2852 |
11 |
3.8±1.2 |
7 |
2.4±0.9 |
|
|
|
AF (R+0) |
4672 |
38 |
8.1±1.3 |
16 |
3.4±0.85 |
|
|
|
AF (R+9) |
3147 |
21 |
7.8±1.7 |
10 |
3.2±0.9 |
|
|
2 |
BF (L-14) |
3792 |
19 |
5.0±1.1 |
6 |
1.6±0.6 |
|
|
|
AF (R+0) |
4843 |
47 |
9.7±1.4 |
19 |
3.9±0.9 |
|
|
|
AF (R+114) |
3604 |
27 |
7.5±1.4 |
16 |
4.4±1.1 |
|
|
*) Blood sampling either before (BF) or after flight. For instance, 14 days before launch (L-14) or on the day of return (R+0). |
|
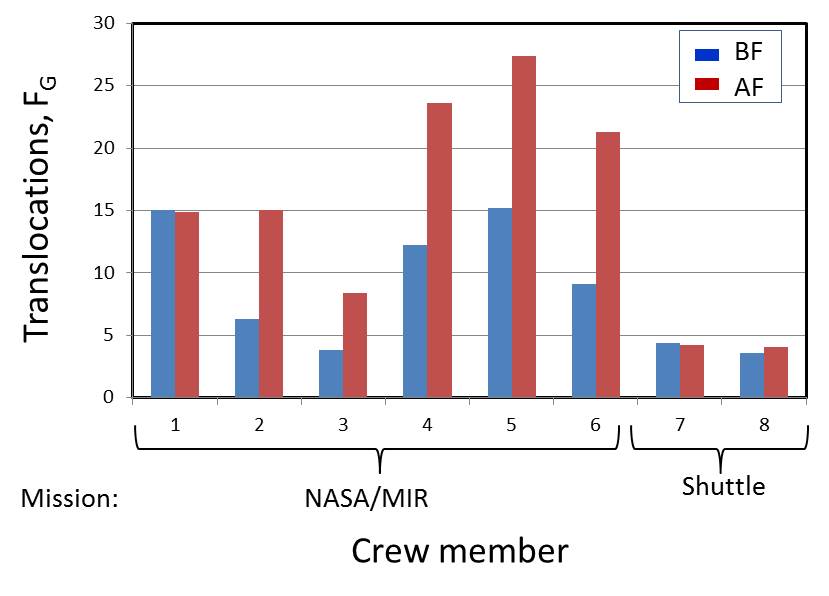
| [2] NASA/MIR and Space Shuttle missions: Analysis by FISH painting method,
George et al. 2001 |
Reference
George, K., Durante, M., Wu, H., Willingham,
V., Badhwar, G and Cucinotta, F. A. (2001): Chromosome aberrations in the
blood lymphocytes of astronauts after space flight. Radiat. Res., 156:731-738.
Chromosome aberration analysis (conventional Giemsa method)
Chromosome aberrations were studied
by FISH painting method for 6 crew members involved in the long-term NASA/MIR
mission and 2 crew memebrs of a short-term shuttle mission. Except for
crew #1 and shuttle members (#7, #8), translocation frequencies were significantly
increased in the post-flight samples as compared to the pre-flight samples.
|
Crew |
Blood |
Cells |
Chromosome |
No. of simple |
No. of complex |
Total no. of |
Genome equivalent frequency****, FG |
|
|
member* |
sampling** |
scored |
painted |
translocations |
exchanges*** |
exchanges |
Total echanges |
Translocations |
|
|
1 |
BF |
4,404 |
1+2 |
19 |
1 |
24 |
(18.9±3.81)×10-3 |
(15.0±3.5)×10-3 |
|
|
|
AF (day 10) |
6,556 |
1+2 |
27 |
7 |
42 |
(22.4±3.5)×10-3 |
(14.9±2.8)×10-3 |
|
|
2 |
BF |
1,892 |
1,2+4 |
5 |
1 |
6 |
(7.6±3.2)×10-3 |
(6.3±2.8)×10-3 |
|
|
|
AF (day 12) |
4,677 |
2+1 |
20 |
2 |
23 |
(17.1±3.5)×10-3 |
(15.0±3.5)×10-3 |
|
|
3 |
BF |
3,995 |
2+4 |
4 |
0 |
4 |
(3.8±1.9)×10-3 |
(3.8±1.9)×10-3 |
|
|
|
AF (day 0) |
4,056 |
2+4 |
9 |
2 |
11 |
(10.3±2.7)×10-3 |
(8.4±2.7)×10-3 |
|
|
|
AF (day 240) |
4,745 |
2+1 |
14 |
2 |
18 |
(13.3±3.1)×10-3 |
(10.1±2.8)×10-3 |
|
|
4 |
BF |
3,792 |
2+4 |
12 |
3 |
17 |
(17.1±4.2)×10-3 |
(12.2±3.4)×10-3 |
|
|
|
AF (day 9) |
4,843 |
2+4 |
30 |
3 |
38 |
(29.6±4.9)×10-3 |
(23.6±4.2)×10-3 |
|
|
|
SF (day 114) |
3,604 |
2+4 |
20 |
0 |
23 |
(24.3±4.9)×10-3 |
(20.914.68)×10-3 |
|
|
5 |
BF |
742 |
2+4 |
3 |
2 |
5 |
(25.5±11.4)×10-3 |
(15.2±8.7)×10-3 |
|
|
|
AF (day 9) |
2,630 |
2+4 |
19 |
0 |
21 |
(30.43±6.5)×10-3 |
(27.4±6.5)×10-3 |
|
|
6 |
BF |
2,852 |
2+4 |
7 |
1 |
8 |
(10.6±3.8)×10-3 |
(9.1±3.4)×10-3 |
|
|
|
AF (day 0) |
4,672 |
2+4 |
26 |
1 |
30 |
(24.3±4.6)×10-3 |
(21.3±4.2×10-3 |
|
|
|
AF (day 9) |
3,147 |
2+4 |
13 |
1 |
19 |
(22.8±5.3)×10-3 |
(15.6±4.2)×10-3 |
|
|
7 |
BF |
2,962 |
1,2+5 |
5 |
1 |
7 |
(6.2±2.3)×10-3 |
(4.4±1.8)×10-3 |
|
|
|
AF (day 0) |
4,287 |
1,2+5 |
7 |
1 |
10 |
(6.0±1.8)×10-3 |
(4.2±1.6)×10-3 |
|
|
8 |
BF |
712 |
1,2+5 |
1 |
0 |
1 |
(3.6±3.6)×10-3 |
(3.6±3.6)×10-3 |
|
|
|
AF (day 0) |
2,529 |
1,2+5 |
4 |
0 |
4 |
(4.1±2.1)×10-3 |
(4.1±2.1)×10-3 |
|
|
*) Crew members 1-6 were involved in NASA/MIR mission for 3-4 months. Crew members 7 and 8 were invloved in short-term (10 day) shuttle mission. |
|
|
**) Blood sampling either before (BF) or after (AF) flight. For instance, AF (day 0) indicates that sampling was made on the day of return. |
|
|
***) Complex exchanges include unbalanced exchanges, insertions and three exchanges on one copy of painted chromosome. |
|
|
|
Only one typical "rogue" cells was found in a total of 26,931 cells analysed by FISH painting in AF samples from long-term NASA/MIR crew members (members 1-6). |
|
|
****) Genome equivalent frequency was obtained by Fp=2.05[fp(1-fp)+fp1fp2+fp1fp3+fp2fp3]FG, a modified version of Lucus et al. (Cytogenet. Genome Res., 60:255-256, 1992) |
. |
| [3] ISS missions: Analysis by FISH painting method, George et al. 2013 |
Reference
George, K., Rhone, J., Beitman, A. and Cucinotta, F. A. (2013): Cytogenetic damage in the blood lymphocytes of astronauts: effects of repeat long-duration space mission. Mutation Res., 756:165-169.
Chromosome
aberration analysis
Chromosome aberration analyses were carried
out by FISH painting method on 5 crew members involved in the ISS mission
for before and after consequtive long-term mission. The 1st post-flight
samples were collected 1-2 weeks after the mission, and for the most crews
a follow-up samples were collected 6-18 months later. Part of blood samples
was irradiated in vitro with gamma-rays to see the dose-response relationships
amenable to use aberration-based biological dosimetry.
| Crew |
1st flight |
|
2nd flight |
| Total frequency (±SD×10-3) |
Translocations (±SD×10-3) |
TLD dose |
|
Total frequency (±SD×10-3) |
Translocations (±SD×10-3) |
TLD dose |
| BF |
AF |
BF |
AF |
(mGy) |
|
BF |
AF |
BF |
AF |
(mGy) |
| A |
3.7±1.9 |
10.1±3.0 |
3.7±1.9 |
8.2±2.7 |
56.8 |
|
8.1±1.2 |
9.2±1.8 |
5.2±1.0 |
6.6±1.3 |
30.9 |
| B |
3.9±1.4 |
7.6±1.8 |
2.5±1.1 |
5.0±1.5 |
22.2 |
|
5.0±1.2 |
9.0±1.6 |
4.1±1.1 |
8.1±1.5 |
38.2 |
| C |
5.8±1.7 |
8.2±1.8 |
4.3±1.4 |
5.8±1.5 |
22.7 |
|
7.7±1.3 |
13.2±1.8 |
5.7±1.0 |
8.7±1.5 |
32.7 |
| D |
4.0±1.1 |
7.7±1.3 |
2.0±0.8 |
5.5±1.1 |
33.1 |
|
12.3±2.0 |
17.4±2.3 |
9.2±1.7 |
10.5±1.8 |
33.4 |
| E |
2.2±0.9 |
7.9±1.8 |
0.7±0.5 |
6.7±1.7 |
29 |
|
8.1±1.4 |
11.9±1.8 |
5.9±1.2 |
9.8±1.6 |
42.8 |
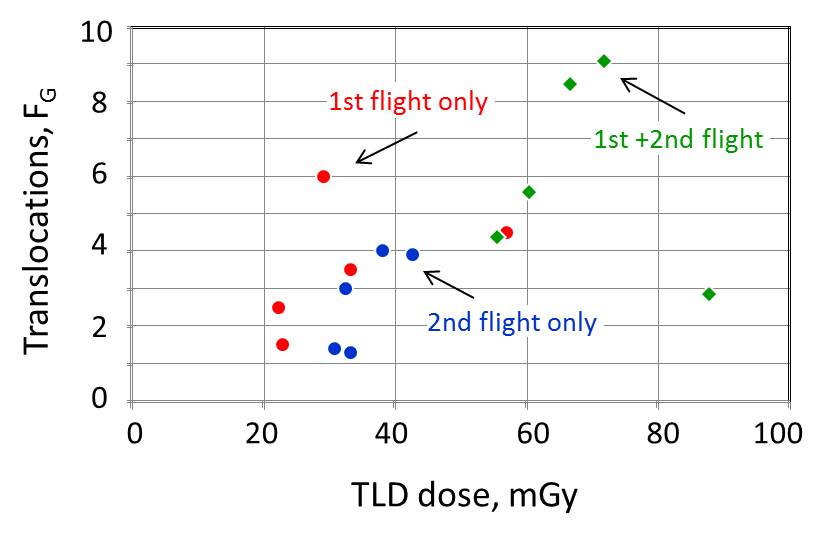
Figure constructed by the Editor: Translocation frequencies against TLD
doses recieved by crews during the respective flights.
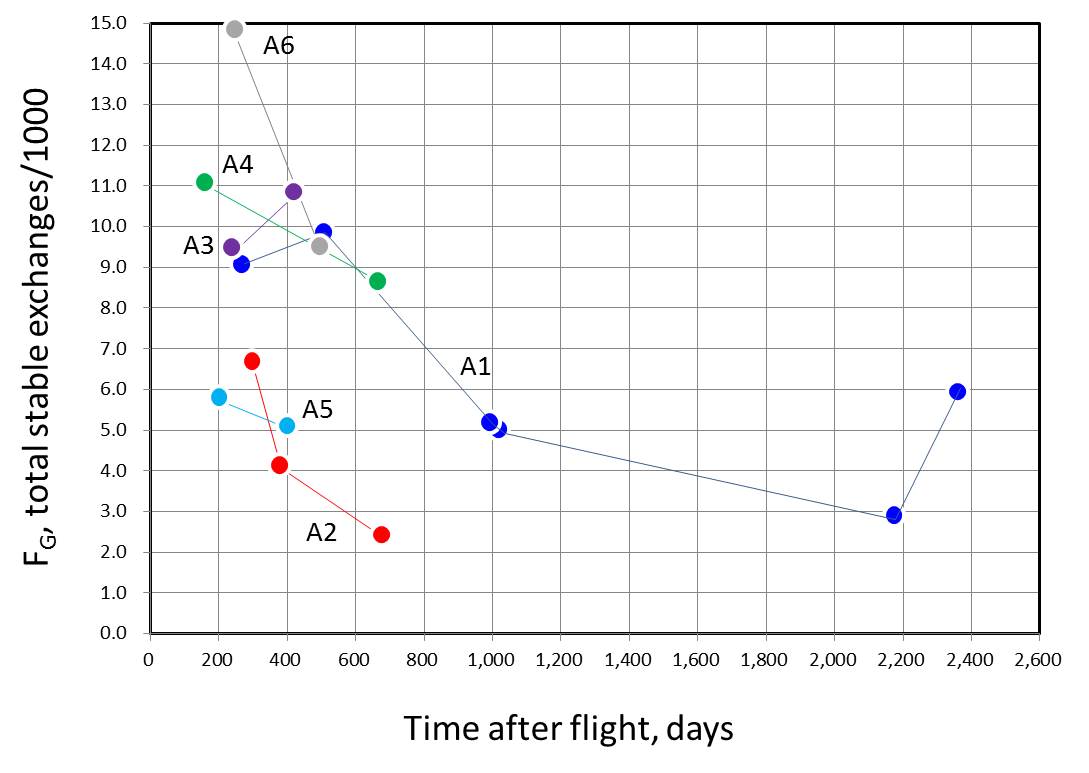
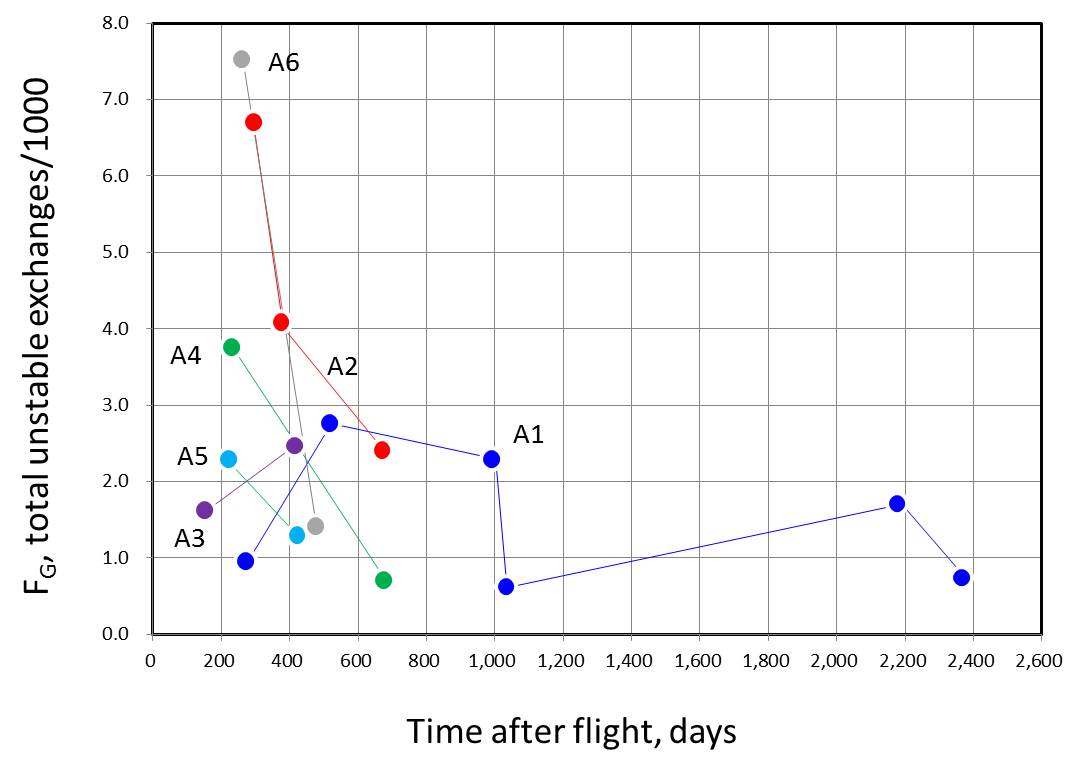
| [4] Stability of aberrations: George et al. 2005 |
Reference
George, K., Willingham, V. and Cucinotta,
F. A. (2005): Stability of chromosome aberrations in the blood lymphocytes
of astronauts measured after space flight by FISH chromosome painting.
Radiat. Res., 164:474-480.
George, K., Chappell, L. J. and Cucinotta,
F. A. (2010): Persistence of space radiation induced cytogenetic damage
in the blood lymphocytes of astronauts. Mutation Res., 701:75-79..
Chromosome
aberration analysis
To seen a stability of chromosome aberrations frequencies in the astronauts, the follow-up measurements of types and frequencies of chromosome aberrations were carried out by FISH-painting technology in the post-flight consecutive blood samples taken from 6 astronauts involved in long-term MIR missions. Significant increase in the chromosome aberrations was observed in the post-flight samples as compared to the pre-flight samples.
However, the aberration frequencies, even
of the stable-type exchanges, tended to decrease with the increase of the
post-flight time lapse (in days or years). This observation raises problem
of the complexity of the use of FISH translocation assay for retrospective
biodosimetry.
|
Astrinaut |
Blood collection* |
Painted |
No. of |
No. of |
No. of |
No. of |
No. of |
Whole genome equivalent exchanges, ×10-3 |
|
|
for |
days |
probe |
cells |
translocations |
dicentrics |
stable complex |
unstable complex |
Total stable |
Total unstable |
|
A1 |
BF |
1 |
2,4 |
3,995 |
4 |
0 |
0 |
0 |
3.7 |
0 |
|
|
AF |
272 |
2,4 |
4,056 |
9 |
0 |
1 |
1 |
9.1 |
0.9 |
|
|
|
513 |
1,2 |
4,745 |
14 |
2 |
0 |
2 |
9.8 |
2.8 |
|
|
|
994 |
1,2,5 |
7,728 |
15 |
6 |
1 |
1 |
5.2 |
2.3 |
|
|
|
1,023 |
1,2,5 |
13,264 |
25 |
3 |
2 |
0 |
5.1 |
0.6 |
|
|
|
2,175 |
1,2,5 |
10,469 |
11 |
4 |
1 |
3 |
2.9 |
1.7 |
|
|
|
2,364 |
1,2,5 |
6,706 |
12 |
2 |
4 |
0 |
6.0 |
0.7 |
|
A2 |
BF |
1 |
1,2,5 |
4,841 |
5 |
0 |
0 |
0 |
2.6 |
0 |
|
|
AF |
295 |
1,2,5 |
7,851 |
21 |
1 |
0 |
4 |
6.7 |
1.6 |
|
|
|
372 |
1,2,5 |
7,930 |
11 |
2 |
2 |
0 |
4.1 |
0.6 |
|
|
|
673 |
1,2,5 |
5,283 |
5 |
1 |
0 |
0 |
2.4 |
0.5 |
|
A3 |
BF |
1 |
1,2,5 |
5,434 |
2 |
0 |
0 |
0 |
1.0 |
0 |
|
|
AF |
230 |
1,2,5 |
5,547 |
19 |
6 |
1 |
2 |
9.4 |
3.7 |
|
|
|
411 |
1,2,5 |
2,169 |
9 |
1 |
0 |
1 |
10.8 |
2.4 |
|
A4 |
BF |
1 |
1,2,5 |
2,259 |
7 |
0 |
4 |
0 |
12.2 |
0 |
|
|
AF |
156 |
1,2,5 |
6,360 |
24 |
4 |
4 |
0 |
11.0 |
1.6 |
|
|
|
667 |
1,2,5 |
3,508 |
10 |
0 |
2 |
0 |
8.6 |
0.7 |
|
A5 |
BF |
1 |
1,2,5 |
5,427 |
9 |
0 |
2 |
0 |
5.3 |
0 |
|
|
AF |
213 |
1,2,5 |
6,696 |
15 |
6 |
0 |
0 |
5.8 |
2.3 |
|
|
|
413 |
1,2,5 |
4,095 |
7 |
2 |
1 |
0 |
5.1 |
1.3 |
|
A6 |
BF |
1 |
1,2,5 |
5,967 |
28 |
0 |
0 |
2 |
11.8 |
2.9 |
|
|
AF |
262 |
1,2,5 |
4,371 |
26 |
0 |
0 |
4 |
14.9 |
7.5 |
|
|
|
489 |
1,2,5 |
1,840 |
6 |
1 |
1 |
1 |
9.5 |
1.4 |
|
*) Blood collection for pre-flight (BF) or post-flight (AF) samples on respective days. |
Figures reconstructed from Table 1 of George et al. (2005). Unstable exchanges
apear to decrease more rapidly than stable exchanges.
Commentary (Editorial):
Reciprocal translocations have been generally considered nonlethal aberrations, and for this reason attempt has been made to use translocations in blood T-lymphocytes for retrospective biological dosimetry. They include cells with only stable-type rearrangements, Cs-cells, or the genome-equivalent frequencies of translocations, FG-Tr, extrapolated from those involved in HISH painted chromosomes,
Fp-Tr, as surrogate markers. However, recently cytogenetic data,
including this paper on astronauts, have been accumulated to indicate that they
also decline over time after radiation exposure, and raised a question on the validity
of their use in retrospective biological dosimetry.
The
transient decline of cells with stable-type aberrations, like unstable ones,
has been well documented in persons acutely irradiated to moderate-to-high
doses of radiation, such as in radiotherapy and radiation accidents, in which the
doses are non-homogeneous and thereby (a) recovery of lymphocyte populations
directly influence on the frequencies of aberrant cells, whether they are of
the stable- or unstable-type.
There
are many other possibilities, of which Gardner and Tucker (2002) have recently
reviewed. They include (b) co-occurrence of unstable-type aberrations, such as
dicentrics, rings, minutes, deletions, etc., which escape the detection by
whole chromosome painting; (c) lethal effects of translocations, DNA loss at
the junction of translocation during DNA DSB end-rejoining, and position
effects of translocation; (d) similarly the DNA loss may occur at the junction
of DSB end-joining resulting in the morphological restitution of breaks. Incidentally,
no significant increase in complex and intrachromosomal exchanges have been
found in post-flight lymphocytes of astronauts involved in short- or long-term
space flight (Horstmann et al. 2005). In addition, (e) the effects of blunting
immune response and its recovery, which are often the case in cancer bearing
patients and astronauts engaged in long-term space missions. In cancer patients
received radiotherapy, the lymphocyte response to PHA dramatically recovers by
washing lymphocytes by culture medium prior to culture, and moreover this
stimulatory recovery is accompanied by an increase of chromosome aberration
frequencies (Matsubara et al. 1980). The mechanism of the immune suppression of
cancer bearing and long-term space flight is not clear. But, it may or may not
be relevant to the poor response of astronaut’s lymphocyte to PHA and decline
of aberration frequencies in lymphocytes. The issue remains to be studied.
Literature
cited
Gardner, S. N. and Tucker, J. D. (2002):
The cellular lethality of radiation-induced chromosome translocations in
human lymphocytes. Radiat. Res., 157:539-552.
Horstmann, M., Durante, M., Jahannes, C., Pieper, R. and Obe, G. (2005): Space radiation does not induce a significant increase of intrachromosomal exchanges in astronauts’ lymphocytes. Radiat. Environ. Biophys., 44:219-224.
Matsubara, S., Horiuchi, J., Shibuya, H.
and Sasaki, M. S. (1980): Effects of washing on phytohemagglutinin responsiveness
of lymphocytes from irradiated patients. Acta Radiol. Oncol., 19:45-54.