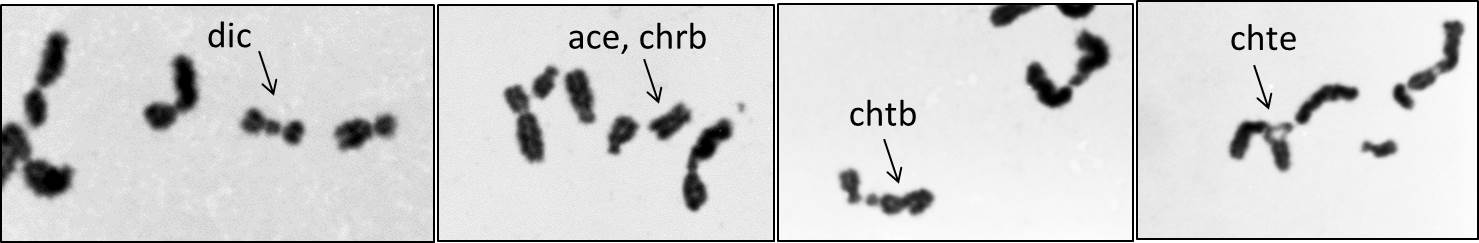
(Chromosome aberrations in human spermatozooa irradiated in vitro as visualized by IVF to hamster oocytes.. Sample picutes are courtesy of Dr. H. Tateno, Biological Sciences, Asahikawa Medical College)
| Sperm: Radiation-induced chromosome aberrations |
| Chromosome aberrations in sperm directly irradiated in vitro |

(Chromosome aberrations in human spermatozooa irradiated in vitro as visualized by IVF to hamster oocytes.. Sample
picutes are courtesy of Dr. H. Tateno, Biological Sciences, Asahikawa Medical
College)
Scenario
To estimate the human genetic risk posed by induced chromosome aberrations, several approaches have been made, e.g., chromosome aberration in somatic cells as surrogates for the genetic effects in germ cells, modelling in the experimental animals such as translocation induction in spermatogonial stem cells.
Recently it became possible to adopt more
direct method to examine the chromosome aberrations in human sperm through
inter-species in vitro fusion of human sperm with eggs from laboratory animals,
such as mouse, Syrian hamster, etc.
The chromosomal effects of radiotherapy on germ cells can be examined with this
technology. The improvement of efficiency of the inter-species in vitro
fertilization (IVF) is in progress because the efficiency of the IVF has been
thought to influence the recovery of primary DNA damage into chromosome
aberrations. In particular, during spermiogenesis (in epididymis), the DNA
associated histone is replaced by –SS- rich protamine. And DNA damage is not
repaired in the sperm until fertilization, where the DNA damage can be repaired
by repair enzymes brought by egg. The unrepaired DNA damage is the subject to
the chemical hydrolysis (storage effects). Recently, several experiments have
shown that the method became relatively stable and consistent results were
obtained for human spermatozoa by inter-species IVF. The sperm assay may call
attention not only to the consequences of radiation exposure to sperm but also to
the genome alterations incurred by radiation during meiotic process that are
later inherited by sperm.
| References |
|
Brandriff,
B. F., Gordon, L. A., Ashworth, L. K. and Carraro, A. (1988): Chromosomal
aberrations induced by in vitro
irradiation: Comparison between sperm and lymphocytes. Environ. Mol. Mutagen.,
12:167-177. |
| . | ||||||||||||||||||
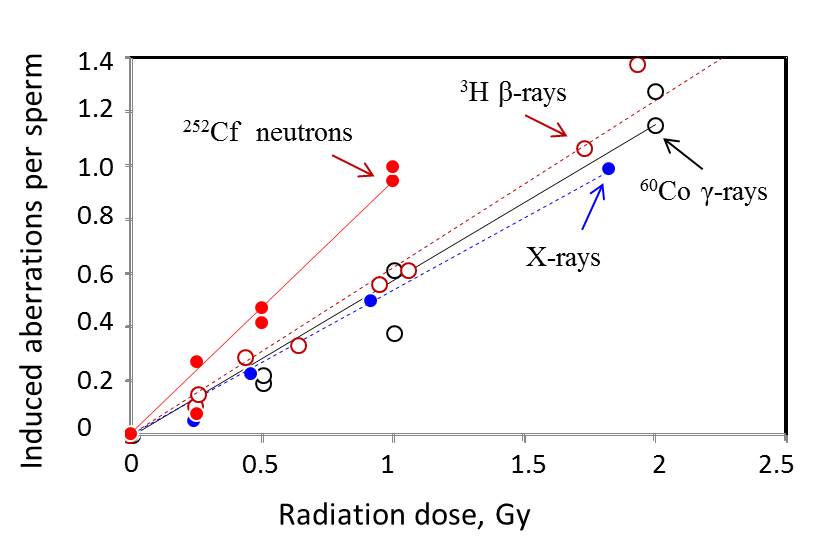
| 60Co gamma-rays (Tateno et al., Int. J. Radiat. Res., 70:229-235, 1996) | 3H β-rays (Kamiguchi et al. Mutation Res., 22:125-131, 1990) | |||||||||||||||||
| Donor | Dose | No. of sperm | Chromosome-type aberrations | Chromatid-type aberrations | Total* | Donor | Dose*** | No. of sperm | Chromosome-type aberrations | Chromatid-type aberrations | Total | |||||||
| (Gy) | analyzed | Breaks | Exchanges | Breaks | Exchanges | No. (per spermatozoon) | (Gy) | analyzed | Breaks** | Exchanges | Breaks** | Exchanges | No. (per spermatozoon) | |||||
| A | 0 | 384 | 49 | 2 | 6 | 4 | 61 | - | 0 | 1,290 | 149 | 1 | 51 | 8 | 209 | |||
| 0.5 | 181 | 53 | 7 | 7 | 2 | 69 (0.195) | 0.25 | 118 | 20 | 2 | 8 | 2 | 32 (0.109) | |||||
| 1 | 201 | 129 | 7 | 15 | 4 | 155 (0.612) | 0.26 | 260 | 63 | 4 | 13 | 2 | 82 (0.153) | |||||
| 2 | 122 | 140 | 7 | 13 | 15 | 175 (1.276) | 0.44 | 204 | 66 | 3 | 20 | 3 | 92 (0.289) | |||||
| B | 0 | 161 | 23 | 2 | 6 | 3 | 34 | 0.64 | 246 | 96 | 4 | 18 | 4 | 122 (0.334) | ||||
| 0.5 | 115 | 43 | 2 | 1 | 4 | 50 (0.224) | 0.95 | 214 | 108 | 8 | 30 | 8 | 154 (0.558) | |||||
| 1 | 169 | 76 | 7 | 7 | 10 | 100 (0.381) | 1.06 | 380 | 227 | 10 | 42 | 14 | 293 (0.609) | |||||
| 2 | 114 | 117 | 14 | 17 | 7 | 155 (1.149) | 1.73 | 41 | 36 | 5 | 8 | 1 | 50 (1.058) | |||||
| 1.93 | 202 | 229 | 21 | 46 | 13 | 309 (1.368) | ||||||||||||
| 220 kVp X-rays (Kamiguchi et al., Mutation Res., 228:133-140, 1990) | 3.74 | 177 | 291 | 33 | 78 | 29 | 431 (2.267) | |||||||||||
| Donor | Dose | No. of sperm | Chromosome-type aberrations | Chromatid-type aberrations | Total | |||||||||||||
| (Gy) | analyzed | Breaks** | Exchanges | Breaks** | Exchanges | No. (per spermatozoon) | 252Cf neutrons (contaminated gamma=33 %) (Tateno et al. Int. J. Radiat. Res., 70:229-235, 1996) | |||||||||||
| 0 | 2,097 | 277 | 16 | 34 | 53 | 380 | Donor | Dose | No. of sperm | Chromosome-type aberrations | Chromatid-type aberrations | Total | ||||||
| 0.23 | 491 | 95 | 0 | 7 | 11 | 113 (0.049) | (Gy) | analyzed | Breaks | Exchanges | Breaks | Exchanges | No. (per spermatozoon) | |||||
| - | 0.45 | 543 | 165 | 11 | 23 | 21 | 220 (0.224) | A | 0 | 165 | 32 | 2 | 2 | 2 | 38 | |||
| 0.91 | 819 | 455 | 19 | 46 | 35 | 555 (0.496) | 0.5 | 110 | 56 | 7 | 3 | 4 | 71 (0.415) | |||||
| 1.82 | 1,009 | 921 | 50 | 107 | 79 | 1175 (0.983) | 1 | 53 | 46 | 6 | 10 | 0 | 62 (0.940) | |||||
| B | 0 | 111 | 28 | 2 | 4 | 2 | 36 | |||||||||||
| 137Cs gamma-rays (Brandriff et al. Env. Mol. Mutagenesis, 12:167-177, 1988) | 0.25 | 142 | 61 | 9 | 8 | 6 | 84 (0.267) | |||||||||||
| Donor | Dose | No. of sperm | Chromosome-type aberrations | Chromatid-type aberrations | Total | 0.5 | 88 | 54 | 4 | 10 | 5 | 73 (0.505) | ||||||
| (Gy) | analyzed | Breaks** | Exchanges | Breaks** | Exchanges | No. (per spermatozoon) | C | 0 | 184 | 33 | 2 | 0 | 1 | 36 | ||||
| 0 | 121 | 4 | 0 | 1 | 1 | 6 | 0.25 | 259 | 94 | 8 | 12 | 4 | 118 (0.260) | |||||
| 1 | 97 | 16 | 3 | 0 | 0 | 19 (0.146) | 1 | 152 | 119 | 15 | 19 | 28 | 181(0.995) | |||||
| - | 2 | 108 | 36 | 6 | 0 | 2 | 44 (0.358) | *) Numbers in parentheses represent net induced frequencies | ||||||||||
| 4 | 121 | 97 | 32 | 0 | 1 | 130 (1.025) | **) Bereaks include "breaks", "fragments", "deletions" and "gaps". "Gaps" are less than 1 % of total breaks. | |||||||||||
| ***) Sperm was irradiated in THO-containing BWW medium. Doses assume electron equibrium between medium and sperm. | ||||||||||||||||||
| . | ||||||||||||||||||
| Chromosome aberrations in sperm of healthy and irradiated men (in vivo) |
[1] Spontaneous aberration frequencies in sperm of healthy men
| . | ||||||||||||||||
| Authors | No. of | No. of sperm | Chromosome abnormalities | |||||||||||||
| donors | analysed | chrb | ace | del | chrg | chre | mar+ | chtb | chtg | chte | Hyperhaploid | Hypohaploid | Multiple | |||
| Brandriff et al. 1984 | 4 | 909 | 37 | 11 | 0 | 0 | 1 | 0 | 6 | 0 | 8 | 5 | 9 | 0 | ||
| Martin et al. 1987 | 30 | 1,582 | 35 | 30 | 4 | 11 | 12 | 5 | 3 | 12 | 11 | 20 | 53 | 2 | ||
| . | ||||||||||||||||
[2] Chromosome aberration frequencies in sperm of cancer patients after
radiotherapy
(Sperm
may not necessarily be the prime target presenting chromosome aberrations
in the sperm assay for in vivo irradiation because the sperm production is often preceeded by the depression
of sperm count in patients received radiotherapy)
| Chromosome aberration studies after radiotheray (RT); Martin et al., Mutation Res., 174:219-225, 1986; Mutation Res., 226:21-30, 1989.. | |||||||||||||
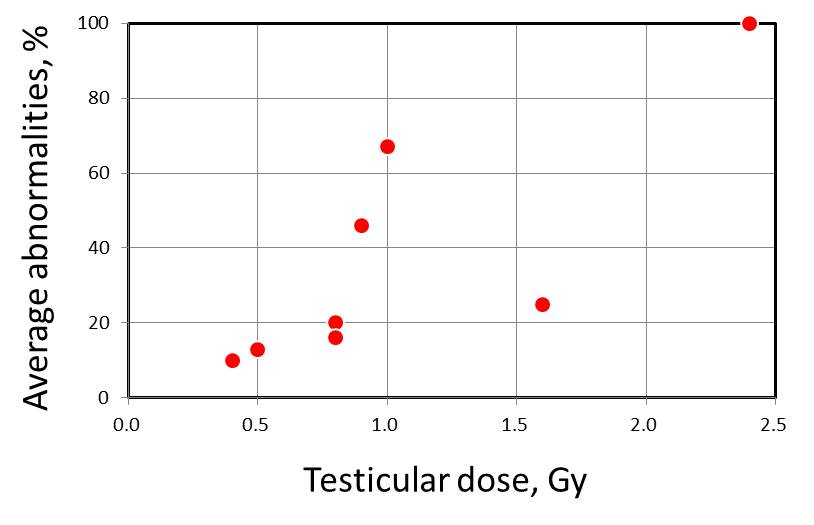
| Patient ID | Age | Cancer | Total radiation | Testicular | Abnormal complements/total complements at indicated time of sperm sampling* | Average abnormality (%) | |||||||
| (year) | dose (Gy) | dose (Gy) | Pre-RT | 1 mo | 3 mo | 12 mo | 24 mo | 36 mo | in post-RT | ||||
| 3 | 31 | Seminoma | 30 | 0.4 | - | - | 0/1 | 0/6 | 6/47 | 1/16 | 10 | ||
| 5 | 20 | Lymphoma | 61.6 | 0.5 | - | - | - | - | 0/2 | 2/13 | 13 | ||
| 11 | 28 | Seminoma | 30+25 | 0.6 | 0/1 | - | - | - | - | - | |||
| 1 | 29 | Seminoma | 30 | 0.8 | - | - | - | - | - | 2/10 | 20 | ||
| 7 | 24 | Semonoma | 30 | 0.8 | - | - | - | - | 1/6 | 4/25 | 16 | ||
| 2 | 40 | Seminoma | 30 | 0.9 | - | - | - | - | - | 5/11 | 46 | ||
| 8 | 29 | Seminoma | 30 | 1.0 | - | - | - | - | - | 2/3 | 67 | ||
| 9 | 22 | Seminoma | 30 | 1.3 | 0/3 | - | - | - | - | - | |||
| 6 | 19 | Teratoma | 40 | 1.6 | 0/1 | - | - | - | - | 1/4 | 25 | ||
| 12 | 20 | Hodgkins | 40 | 2.4 | - | - | 1/1 | - | - | - | 100 | ||
| 4 | 47 | Seminoma | 30 | 3.1 | 0/4 | - | - | - | - | - | |||
| 10 | 39 | Rectal cancer | 45 | 5.0 | - | - | - | - | - | - | |||
| 13 | 41 | Muduloblastoma | 41+33+36+20 | ND | - | - | - | - | - | 1/4 | 25 | ||
| *) Abnormalities include aneuploidy (n=36), chromosome-type and chromatid-type structural abnormalities (n=36) in 51 abnormal cellsfound in 337 cells analysed. | |||||||||||||
| . | |||||||||||||