
| Atomic Bombing (A-bomb) in Hiroshima and Nagasaki |
 |
| Early days of Hiroshima and Nagasaki after atomic bombing (adopted from 6 August 2015 issue of the Asahi Daily News |
 |
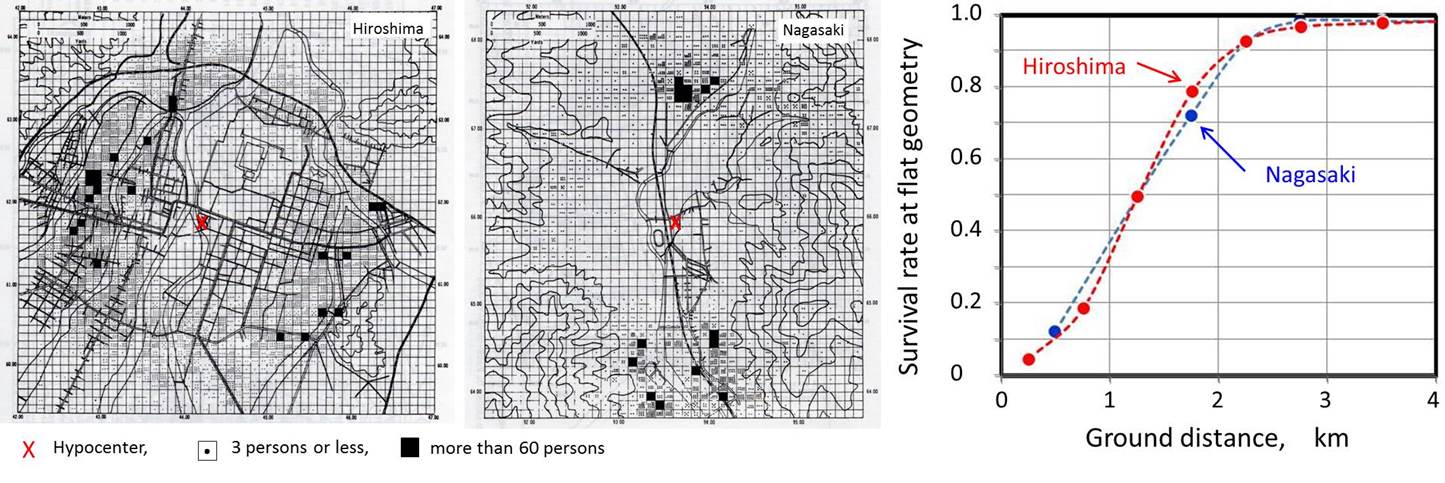
| A. Density map of survivors resitered in LSS cohort to whom T65D doses have been assigned [1]. B. Survival rate (inverese of death rate) as of 10 August 1946 [2]. |
| Scenario [1-5] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fission-type atomic bombs (A-bomb) were detonated over Hiroshima and Nagasaki cities on August 1945. The damage in the cities was caused by a combination of burns, concussions and radiation. Out of a total of about 660,000 persons in the two cities, about 200,000 persons died by the end of 1945, and about 340,000 persons died during the first 5 years [1, 2]. To examine the medical and biological effects of atomic bombings, the Atomic Bomb Casualty Commission (ABCC) was established in Hiroshima and Nagasaki on 26 November 1946 with funding by the U. S. Atomic Energy Commission through reputation of U. S. National Academy of Science. The organization was later succeeded by the US-Japan binational funding organization, Radiation Effects Research Foundation (RERF), in 1975. In the nationwide survey, National Census, conducted in 1950, a total of 284,000 survivors were identified throughout the country. A group of about 110,000 subjects was sampled from those exposed survivors (< 2.5 km from hypocenter) and non-exposed controls (2.5-10 km from hypocenter) who were resident in Hiroshima and Nagasaki. This group, established in 1958, was called Life Span Study (LSS) cohort, comprising the exposed and control groups of comparable in size, sex and age except for exposure, and periodically followed up for the mortality. When the subject died, the cause of death has been confirmed by autopsies. The LSS cohort includes a subset of about 20,000 people, called Adult Health Study (AHS) cohort, comprising 5,000 exposed within 2 km and showed acute radiation symptoms, 5000 exposed within 2 km but no acute radiation symptom, 5000 who were 3 km or far, and 5000 people who were not in city at the time of bombing. Detailed biennial medical examinations have been carried out in this cohort. In addition, the in utero exposed group (about 2,800 subjects), and F1 mortality study cohort (77,000 subjects) have been set. The cytogenetics program was initiated in 1968
and analysis have been carried out in samples from the cohort of AHS group
and children born to the exposed parents (F1 and in utero
cohorts). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Dosimetry [6, 7] |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
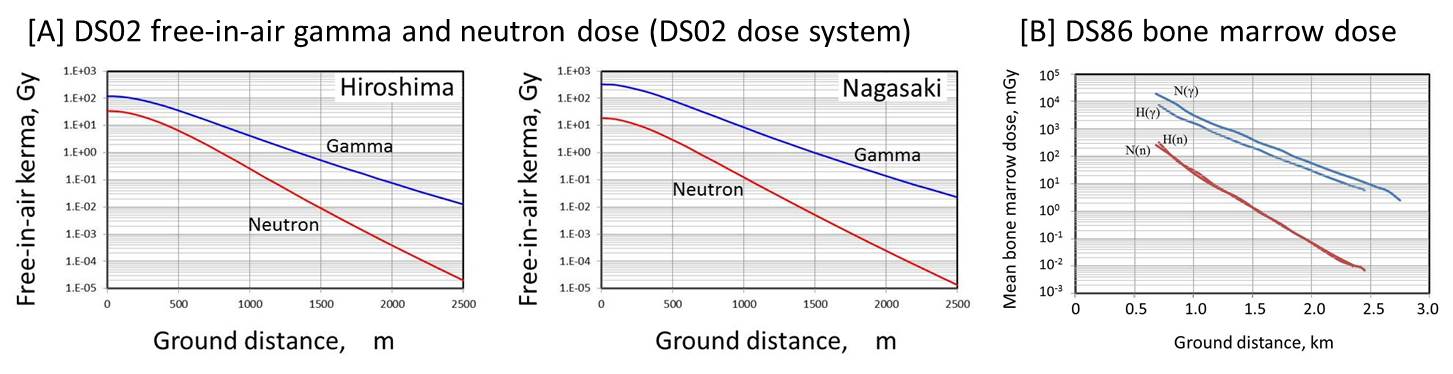
The early dose estimates for survivors, called tentative 1957 dose (TD57D) have been made by the experimental data of the A-bomb explosion in the Nevada Test Site. This was revised to a TD65D in 1965. In these early dosimetry systems, the neutron component was very comparable to that of gamma-rays in Hiroshima. Based on the accumulation of new output spectra and measurement data on activation, the dosimetry system was revised to DS86, dosimetry system in 1986, and further with minor revisions to the current DS02 dosimetry system in 2002 [ref. 6] The A-bomb radiation is a mixture of gamma-rays and neutrons. In the evaluation of medical and biological effect of A-bomb radiation, including chromosome damage, the relative biological effectiveness (RBE) of neutrons is critical. In the A-bomb survivors, historically the neutrons have been weighted by constant RBE of 10. That is, the dose to survivors (D in Sv) is expressed by D=Dg+10×Dn, Dγ and Dn are gamma-ray and neutron dose in tissue kerma (in Gy), respectively. Recently, the doses are further modified by so-called adjustment factor (ε), like in D=ε(Dg+10×Dn). The adjustment factor is the dose modification factor derived from the limits of random dosimetry errors, and dependent on dose and differs by city. The modified dose is called ‘survivor dose’ [ref. 7 for review]. For organ dose, DS86 bone marrow dose is shown below because the chromosome aberration analysis has been so far discussed on the basis of DS86 dosimetry system. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Chromosome analysis in survivors: Studies in RERF [8-12] |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Blood samples were collected the Adult Health
Study (AHS) cohort between 1968 and 1980 from proximally exposed survivors
(within 2 km from hypocenter), and their controls who were non-exposed
(NIC) or exposed distally (2.5 km or more away from hypocenter). The dosimetry
system referred to here was Free-in-Air (FIA) kerma in DS86 dosimetry system. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| . | ||||||||||||
| . | [A] Blood sampling in 1968-1980: Awa, A. A. (1991), J. Radiat. Res., Supplement, 265-274. | |||||||||||
| DS86 | Hiroshima | Nagasaki | ||||||||||
| dose group | Mean dose | No. of | No. of | Aberrant | Mean dose | No. of | No. of | Aberrant | ||||
| (Gy) | (Gy) | subjects | cells | cells** | (Gy) | subjects | cells | cells** | ||||
| Distantly exposed* | 0-0.004 | 0 | 362 | 34,299 | 363 | 0 | 251 | 24,539 | 345 | |||
| Proximally exposed | 0.005-0.09 | 0.048 | 13 | 1,188 | 13 | 0.7 | 19 | 1,853 | 22 | |||
| 0.10-0.49 | 0.295 | 60 | 5,624 | 105 | 0.299 | 48 | 4,584 | 83 | ||||
| 0.50-0.99 | 0.779 | 90 | 8,697 | 436 | 0.738 | 49 | 4,768 | 140 | ||||
| 1.00-1.99 | 1.43 | 152 | 14,500 | 1,309 | 1.42 | 61 | 5,990 | 433 | ||||
| 2.00-2.99 | 2.45 | 83 | 8,057 | 1,340 | 2.39 | 23 | 2,215 | 369 | ||||
| 3.00-3.99 | 3.37 | 28 | 2,643 | 577 | 3.45 | 6 | 600 | 111 | ||||
| *) Distantly exposed group include persons who were not in city (NIC) at the time of bombing. | ||||||||||||
| **) The aberrations involed were mostly stable-type aberration (reciprocal translocations and inversions), and unstable aberrations were less than 10% of total aberrations. | . | |||||||||||
|
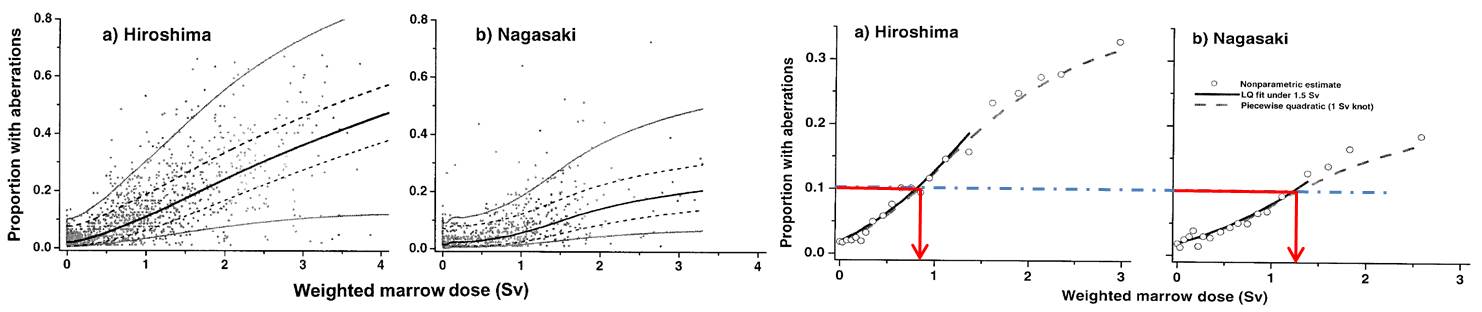
In the later studies, chromosome aberration frequencies were plotted against bone marrow dose, the difference in the dose-response curves between Hiroshima and Nagasaki did not disappeared as shown by the difference in the equi-effective doses between two cities (see below). For alternative implication of dose-response, see M. S. Sasaki et al. |
 |
| Chromosome examination in F1 offspring: Studies in RERF [13, 14, 15] |
|
The analysis of chromosome constitutions
in the F1 children born to
A-bomb survivors was initiated in 1967 [5]. This cohort includes children born
between 1 May 1946 and 31 December 1958 to parents, one or both of whom were
residents of Hiroshima and Nagasaki ATB. The samples were later expanded to
include children who were born after 1959 through the end of 1972. The later
samples constitute about 10% of the total. |
| Hiroshima | Nagasaki | Two cities | ||||||||||||||||||
| Types of chromosome abnormalities and number of cases examined | Controls | Exposed | Controls | Exposed | Controls | Exposed | ||||||||||||||
| Father | Mother | Both | Total | Father | Mother | Both | Total | Father | Mother | Both | Total | |||||||||
| Number of cases examined | 5,112 | 1,274 | 2,838 | 604 | 4,716 | 2,864 | 1,166 | 2,035 | 405 | 3,606 | 7,976 | 2,440 | 4,873 | 1,009 | 8,322 | |||||
| Autosome structural aberrations | Balanced | rob(D/D) | 4 | 2 | 3 | - | 5 | 2 | 2 | - | - | 2 | 6 | 4 | 3 | - | 7 | |||
| rob(D/G) | - | - | 1 | - | 1 | - | 1 | - | 1 | 2 | - | 1 | 1 | 1 | 3 | |||||
| rec | 6 | 3 | 2 | 2 | 7 | 7 | - | - | - | - | 13 | 3 | 2 | 2 | 7 | |||||
| inv | 6 | - | 1 | - | 1 | - | - | - | - | - | 6 | - | 1 | - | 1 | |||||
| Unbalanced | rob | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||||
| rec | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||||
| del | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||||
| supern | - | 1 | - | - | 1 | - | 1 | - | - | 1 | - | 2 | - | - | 2 | |||||
| others | - | 1 | 1 | - | 2 | 2 | - | 1 | - | 1 | 2 | 1 | 2 | - | 3 | |||||
| Sex chromosome abnormalities | Male | XYY | 5 | - | - | - | - | - | 2 | 1 | - | 3 | 5 | 2 | 1 | - | 3 | |||
| XXY | 4 | 1 | 4 | 1 | 6 | 5 | - | 1 | - | 1 | 9 | 1 | 5 | 1 | 7 | |||||
| Mosaic | - | 1 | - | - | 1 | - | - | - | - | - | - | 1 | - | - | 1 | |||||
| others | 2 | - | - | - | - | - | - | 1 | - | 1 | 2 | - | 1 | - | 1 | |||||
| X | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||||
| Female | XXX | 3 | 2 | 1 | 1 | 4 | 1 | 1 | - | - | 1 | 4 | 3 | 1 | 1 | 5 | ||||
| Mosaic | 3 | - | - | 1 | 1 | - | - | 1 | - | 1 | 3 | - | 1 | 1 | 2 | |||||
| others | - | - | - | - | - | 1 | - | - | - | - | 1 | - | - | - | - | |||||
| Notes: (1) All Robertsonian translocations, rob(D/D) and rob(D/G) were familial. | ||||||||||||||||||||
| (2) A balanced reciprocal translocation in one child born to the exposed parentand (Hiroshima) and one in unexposed (Hiroshima) was new mutation. The heritability of others were not determined. | ||||||||||||||||||||
| (3) In the sex chromosome mosaic, one of the controls had 45,X/46,X,r(X) mosaiism. In Nagasaki, a male child born to the exposed mother was 46,XX male. | ||||||||||||||||||||
| (4) Average parental gonadal dose was 0.603 Sv based on DS86 dosimetry system. | ||||||||||||||||||||
|
References |
|
1. Milton, R. C. and Shohoji, T. (1968):
Tentative 1965 radiation dose estimation for atomic bomb survivors. ABCC/RERF
Technical Report TR-1-68. |