Radiation Biology Center, Kyoto University
COE Program: Bioregulation of Radiation Response
---Radiation Effects in a Setting of Life System ---
Advances in Brief (1995-1999)
|
 Radiation Biology Center, founded in 1976, is a national cooperative research institute for the fundamental research on the effects of radiations. Toward the 21st century, major concerns in radiation biology have shifted to the biological effects of low dose and low dose-rate radiations, where there is an increasing need for the dissection based on the fundamental life science. In 1995, the Center was appointed as a Center of Excellence (COE). In this context, the Center started a new research project "Bioregulation of Radiation Response" as a COE program, which covered the areas relating with (1) gene regulation of radiation response, (2) molecular mechanisms of DNA repair, (3) gene regulation of cell cycle, (4) mechanisms of genome stability, and (5) higher ordered regulation. The emphasis has been placed on the fusion of radiation effects and life science as recommended by the External Review Committee, which met in 1995 and 1996. This brochure summarizes some innovative topics in the progress of the COE program performed in 1995-1999.
Radiation Biology Center, founded in 1976, is a national cooperative research institute for the fundamental research on the effects of radiations. Toward the 21st century, major concerns in radiation biology have shifted to the biological effects of low dose and low dose-rate radiations, where there is an increasing need for the dissection based on the fundamental life science. In 1995, the Center was appointed as a Center of Excellence (COE). In this context, the Center started a new research project "Bioregulation of Radiation Response" as a COE program, which covered the areas relating with (1) gene regulation of radiation response, (2) molecular mechanisms of DNA repair, (3) gene regulation of cell cycle, (4) mechanisms of genome stability, and (5) higher ordered regulation. The emphasis has been placed on the fusion of radiation effects and life science as recommended by the External Review Committee, which met in 1995 and 1996. This brochure summarizes some innovative topics in the progress of the COE program performed in 1995-1999.
1. Cloning of a Novel Gene (6-4) Photolyase and Its Molecular Evolution
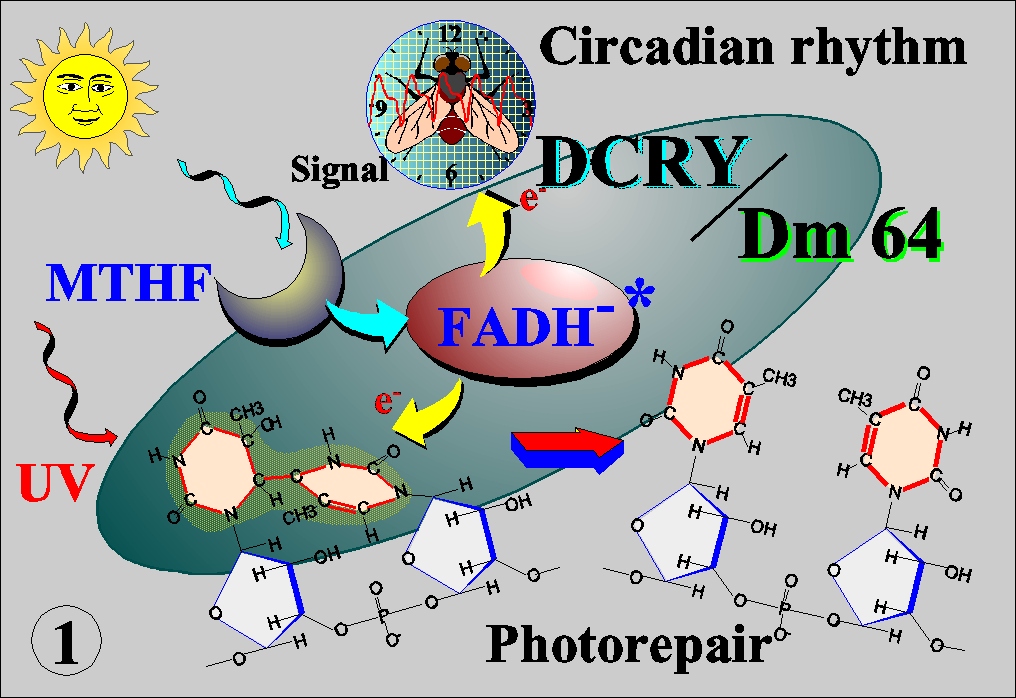
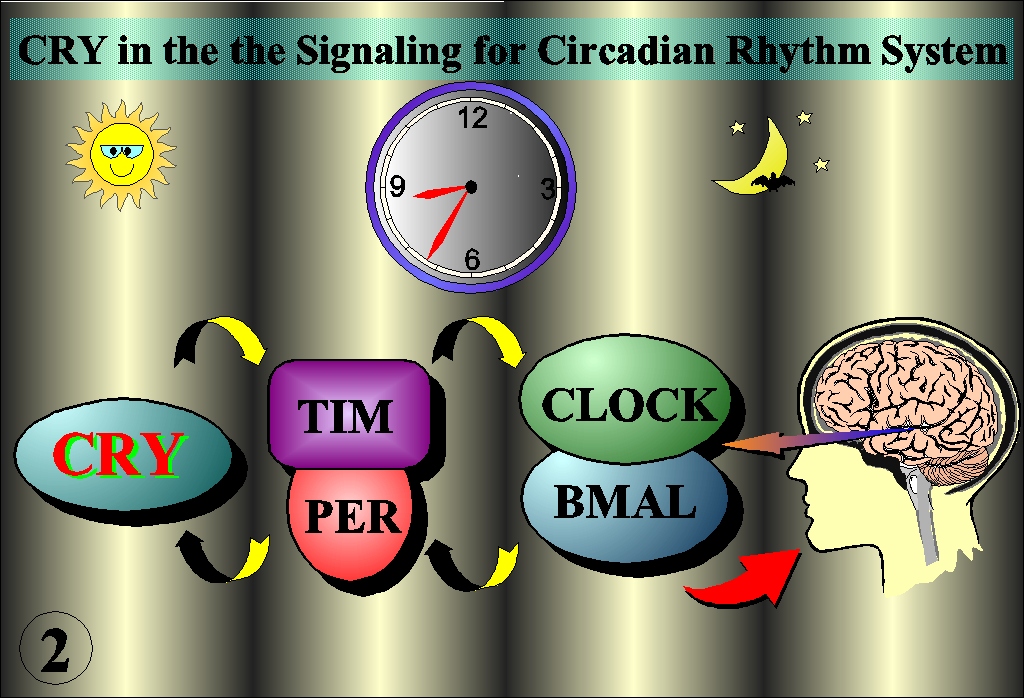
 Photoreactivation is the most efficient system to repair UV-induced DNA damage in that it converts light energy to electron, which in turn directly splits the dimerized bases into original conformation. Unlike cyclobutane pyrimidine dimer (CDP), (6-4) photoproduct has long been believed not to be a substrate of photorepair because of its specific steric structure. However, we identified a protein that specifically binds to (6-4) photoproduct, and finally cloned a gene (6-4) photolyase, Dm64, from Drosophila (Science, 272:109, 1996). Surprisingly, while its high sequence homology to CPD photolyase, the Dm64 also showed a high homology to blue-light photoreceptor (cryptochrome, CRY) in plant. Both have FAD chromophore, which catalyze photorepair in the case of photolyase or transduce photosignal in the case of CRY by transferring electron as a result of energy transfer from a second light-harvesting chromophore, MTHF or 8-HDF (Nucleic Acid Res., 25:764, 1997; J. Biol. Chem., 272:32591, 1997) (Fig. 1). We further cloned genes with high homology to Dm64 from human and Drosophila cells, herein called HsCRY and DCRY, which did not catalyze the photorepair but shared characteristics of CRY(Science, 272:764, 1996; Mut. Res., 384:195, 1997; Genes to Cells, 4:57, 1999). Thus, photolyases and CRY form a structurally and functionally related families which evolved by gene duplication ( J. Mol. Evol., 45:535, 1997)。Furthermore, the CRY was demonstrated to play a role in circadian rhythm (Genes to Cells, 4:57, 1999). Other recent studies indicate that CRY has a pivotal role in the circadian rhythm coupled with other regulator molecules such as TIM/PER and CLOCK/BMAL (Fig. 2). The April issue of Science in this year focused on a molecular diversity of photo-sensing in the repair and circadian rhythm (Fig.3).
Photoreactivation is the most efficient system to repair UV-induced DNA damage in that it converts light energy to electron, which in turn directly splits the dimerized bases into original conformation. Unlike cyclobutane pyrimidine dimer (CDP), (6-4) photoproduct has long been believed not to be a substrate of photorepair because of its specific steric structure. However, we identified a protein that specifically binds to (6-4) photoproduct, and finally cloned a gene (6-4) photolyase, Dm64, from Drosophila (Science, 272:109, 1996). Surprisingly, while its high sequence homology to CPD photolyase, the Dm64 also showed a high homology to blue-light photoreceptor (cryptochrome, CRY) in plant. Both have FAD chromophore, which catalyze photorepair in the case of photolyase or transduce photosignal in the case of CRY by transferring electron as a result of energy transfer from a second light-harvesting chromophore, MTHF or 8-HDF (Nucleic Acid Res., 25:764, 1997; J. Biol. Chem., 272:32591, 1997) (Fig. 1). We further cloned genes with high homology to Dm64 from human and Drosophila cells, herein called HsCRY and DCRY, which did not catalyze the photorepair but shared characteristics of CRY(Science, 272:764, 1996; Mut. Res., 384:195, 1997; Genes to Cells, 4:57, 1999). Thus, photolyases and CRY form a structurally and functionally related families which evolved by gene duplication ( J. Mol. Evol., 45:535, 1997)。Furthermore, the CRY was demonstrated to play a role in circadian rhythm (Genes to Cells, 4:57, 1999). Other recent studies indicate that CRY has a pivotal role in the circadian rhythm coupled with other regulator molecules such as TIM/PER and CLOCK/BMAL (Fig. 2). The April issue of Science in this year focused on a molecular diversity of photo-sensing in the repair and circadian rhythm (Fig.3).
2. Molecular Mechanisms of Dose Recognition and Radioadaptive Response
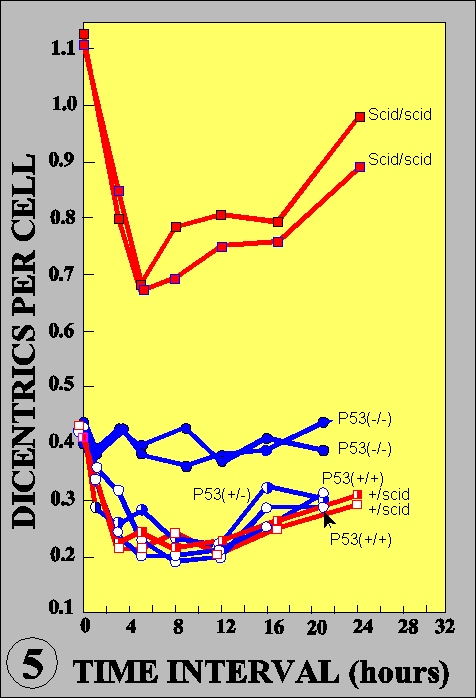
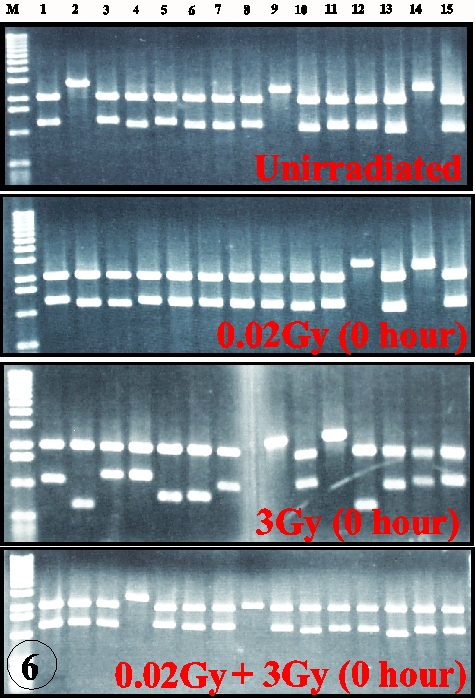
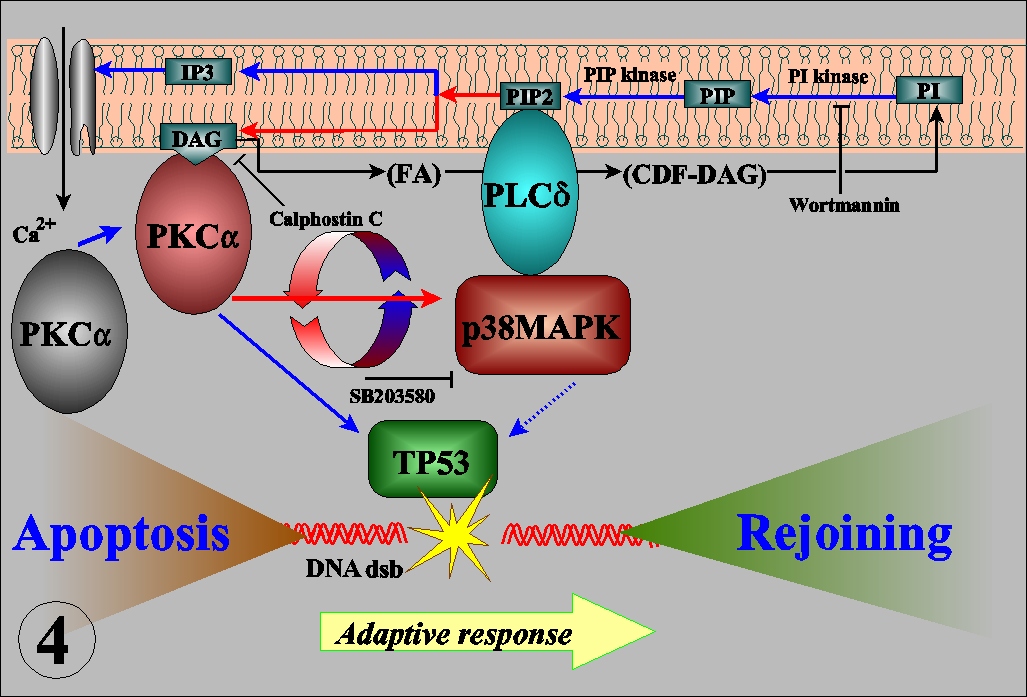 The radioadaptive response is an acquirement of radioresistance of the cells previously irradiated with low dose radiations. Its molecular mechanisms are largely unknown. In cultured mouse cells, adaptive response has a dose limitation up to 100 mSv, continues for more than 20 hours, and renders the cells resistant to mutation induction and cell killing, but not for malignant transformation, by subsequent radiations (Int. J. Radiat. Biol., 68:281, 1995). The kinetics led us to propose a new interpretation for the inverse dose-rate effect of low dose-rate exposures (Mut. Res., 358:207, 1996). The dose recognition and sustained nature of the adaptive response were found to be regulated by a nexus of PKC-p38MAPK-PLC1 feedback signal transduction pathway (Exp. Cell Res., 1999, in press). The activation of PKC continues for long time after low dose irradiation while it is immediately down-regulated after high dose. The activation of PKC is also coordinated with the adaptive response and activation of p38MAPK, which is physically associated with PLC1. Inhibitor of either one of the kinases inhibits activation of another and abrogates adaptive response. The adaptive response is dependent on p53, but not on the ATM or SCID facor (Fig. 5), and furthermore it is associated with the reduction of spontaneous and radiation-induced apoptosis. The in vitro assay for the rejoining of DNA double strand breaks (dsb) indicates that the adaptive response enhances the efficiency and fidelity of the rejoining of DNA dsb (Fig. 6). These lines of evidence suggest that the adaptive response is a reflection of the inducible efficient repair of DNA dsb which is otherwise death signal and substrate for mutation, chromosome aberration and reproductive cell death. Thus, the living organisms seem to have evolved an efficient system to escape from the detrimental effects on their genome.
The radioadaptive response is an acquirement of radioresistance of the cells previously irradiated with low dose radiations. Its molecular mechanisms are largely unknown. In cultured mouse cells, adaptive response has a dose limitation up to 100 mSv, continues for more than 20 hours, and renders the cells resistant to mutation induction and cell killing, but not for malignant transformation, by subsequent radiations (Int. J. Radiat. Biol., 68:281, 1995). The kinetics led us to propose a new interpretation for the inverse dose-rate effect of low dose-rate exposures (Mut. Res., 358:207, 1996). The dose recognition and sustained nature of the adaptive response were found to be regulated by a nexus of PKC-p38MAPK-PLC1 feedback signal transduction pathway (Exp. Cell Res., 1999, in press). The activation of PKC continues for long time after low dose irradiation while it is immediately down-regulated after high dose. The activation of PKC is also coordinated with the adaptive response and activation of p38MAPK, which is physically associated with PLC1. Inhibitor of either one of the kinases inhibits activation of another and abrogates adaptive response. The adaptive response is dependent on p53, but not on the ATM or SCID facor (Fig. 5), and furthermore it is associated with the reduction of spontaneous and radiation-induced apoptosis. The in vitro assay for the rejoining of DNA double strand breaks (dsb) indicates that the adaptive response enhances the efficiency and fidelity of the rejoining of DNA dsb (Fig. 6). These lines of evidence suggest that the adaptive response is a reflection of the inducible efficient repair of DNA dsb which is otherwise death signal and substrate for mutation, chromosome aberration and reproductive cell death. Thus, the living organisms seem to have evolved an efficient system to escape from the detrimental effects on their genome.
3. Role of Homologous Recombination in Genome Integrity
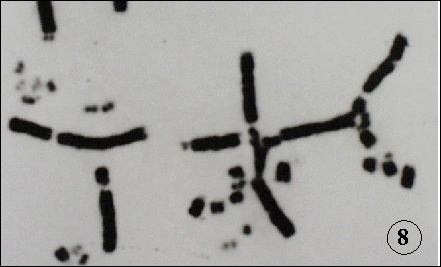
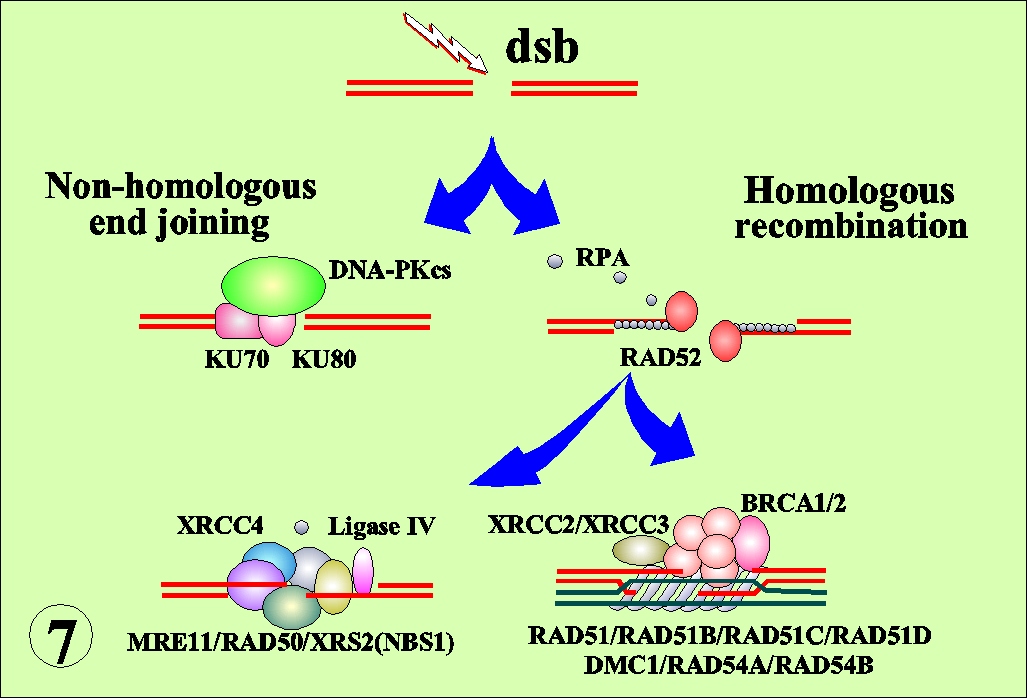 DNA double strand breaks (dsb) are the integral components of the biological effects of radiations. In bacterial system, DNA recombination has been implicated for the repair of dsb. Recent cloning of recombination- related mammalian genes and their disruption in chicken B cells made it possible to analyze recombinational events in radiation effects in higher organisms. DNA dsb may be repaired by two processes; non-homologous end joining (NHEJ) or homologous recombination (HR) (Fig. 7). The disruption of linear order of DNA is manifested as chromosome structural aberrations (Fig. 8). The RAD51-deficient chicken cells accumulate iso-chromatid breaks, indicating that RAD51 plays
an essential role in repairing spontaneously occurring chromosome breaks in DNA replications (EMBO
J., 17:598, 1998). The analysis in KU70/RAD54 double knock-out cells indicates that NHEJ plays a
major role in DNA dsb in G1 phase, which is supplemented by HR pathways in association with DNA replication (EMBO J., 17:5497, 1998). Sister chromatid exchange (SCE) is a switch of a newly synthesized chromatid with its sister. It was evident that KU70 or NHEJ was not involved in SCE formation. However, the spontaneous and
chemical (mitomycin)-induced SCE was suppressed to occur by the disruption of HR-related genes
(Mol. Cell. Biol., 19:5166, 1999). Surprisingly, chromatid aberrations (CA) were also suppressed to
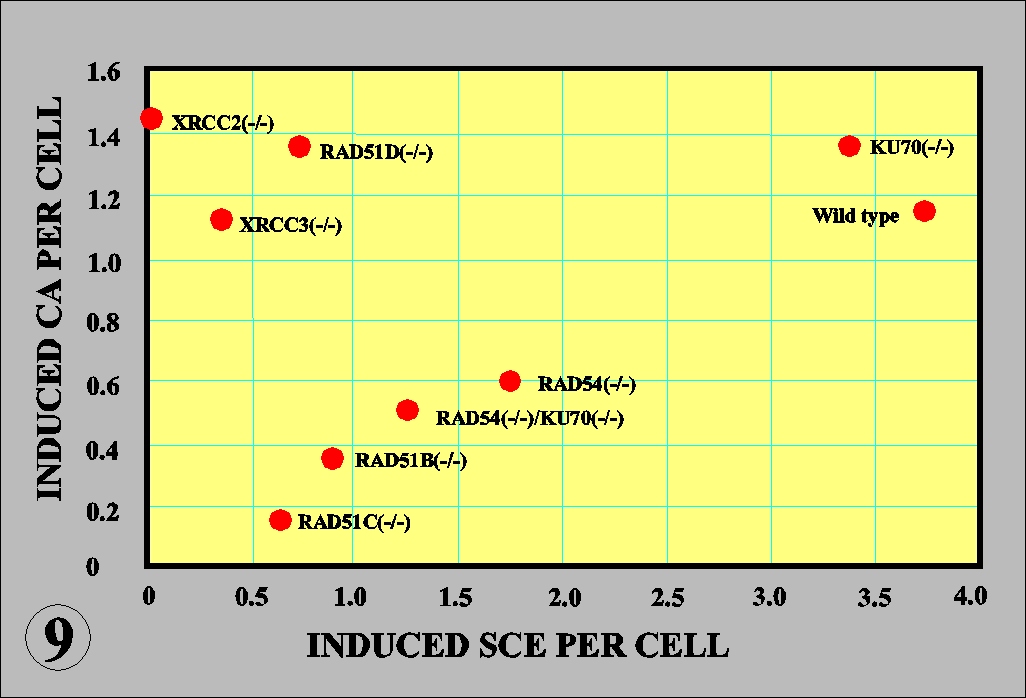
occur in the cells deficient of HR-related genes except for XRCC2/3 and RAD51D genes (Fig. 9). The observations provide the first genetic evidence that SCE and chemical-induced CA are mediated by HR pathway, and moreover show that HR gene families are cooperatively involved in the recombinational repair while each has a distinct role (Fig. 9).
DNA double strand breaks (dsb) are the integral components of the biological effects of radiations. In bacterial system, DNA recombination has been implicated for the repair of dsb. Recent cloning of recombination- related mammalian genes and their disruption in chicken B cells made it possible to analyze recombinational events in radiation effects in higher organisms. DNA dsb may be repaired by two processes; non-homologous end joining (NHEJ) or homologous recombination (HR) (Fig. 7). The disruption of linear order of DNA is manifested as chromosome structural aberrations (Fig. 8). The RAD51-deficient chicken cells accumulate iso-chromatid breaks, indicating that RAD51 plays
an essential role in repairing spontaneously occurring chromosome breaks in DNA replications (EMBO
J., 17:598, 1998). The analysis in KU70/RAD54 double knock-out cells indicates that NHEJ plays a
major role in DNA dsb in G1 phase, which is supplemented by HR pathways in association with DNA replication (EMBO J., 17:5497, 1998). Sister chromatid exchange (SCE) is a switch of a newly synthesized chromatid with its sister. It was evident that KU70 or NHEJ was not involved in SCE formation. However, the spontaneous and
chemical (mitomycin)-induced SCE was suppressed to occur by the disruption of HR-related genes
(Mol. Cell. Biol., 19:5166, 1999). Surprisingly, chromatid aberrations (CA) were also suppressed to
occur in the cells deficient of HR-related genes except for XRCC2/3 and RAD51D genes (Fig. 9). The observations provide the first genetic evidence that SCE and chemical-induced CA are mediated by HR pathway, and moreover show that HR gene families are cooperatively involved in the recombinational repair while each has a distinct role (Fig. 9).
4. Mechanisms of the Damage Surveillance in the Early Stage of Development
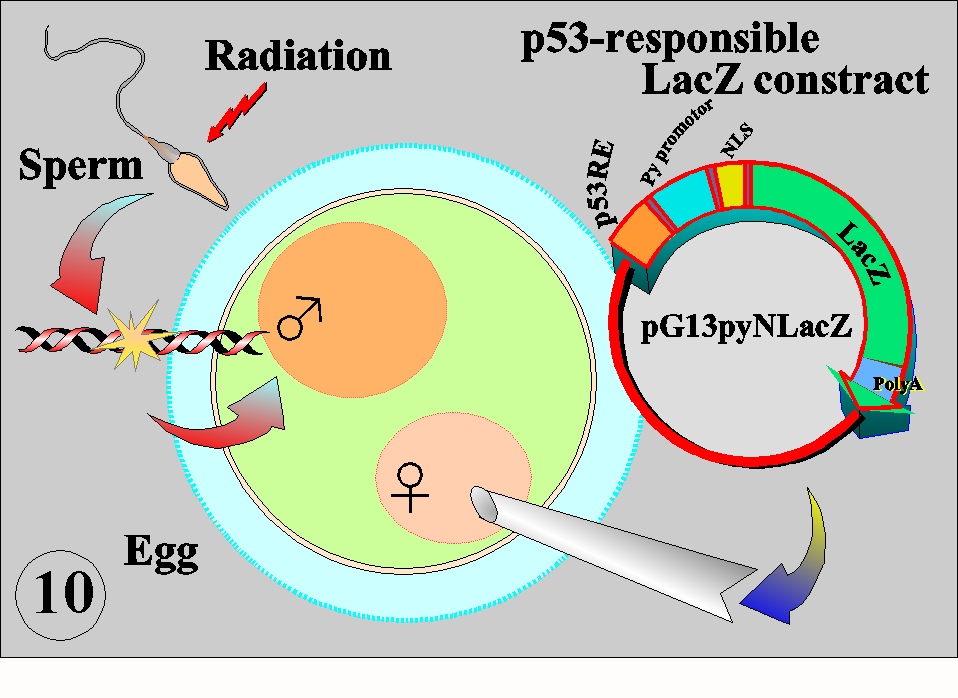 Sperm and egg are carriers of the genome to the next generation. In terms of the damage clearance system, mature sperm constitutes a particular stage in the life cycle because they lack DNA repair activity and therefore oxidative DNA damage due to energy metabolism and other damage inflicted by environmental agents including radiation can not be repaired. The living organisms must have evolved an excellent system to avoid such detrimental effects introduced by sperm. In the experimental system with the mouse eggs, it was found that p53 was playing a key role in the surveillance of the carried-in damage (Fig. 10). The p53 responsible LacZ contruct was injected into
female pronucleus of the egg fertilized by X-irradiated sperm, and the activation of p53 was studied by
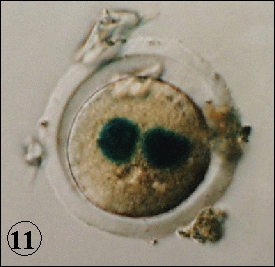
the expression of nuclear localizing LacZ (-galactosidase) on X-gal base. Only when the p53+/+ eggs
were fertilized with X-irradiated sperm, both male and female pronuclei were stained, indicating that the
activation of p53 and translocation of the expressed -galactosidase into nuclei (Fig. 11). In this
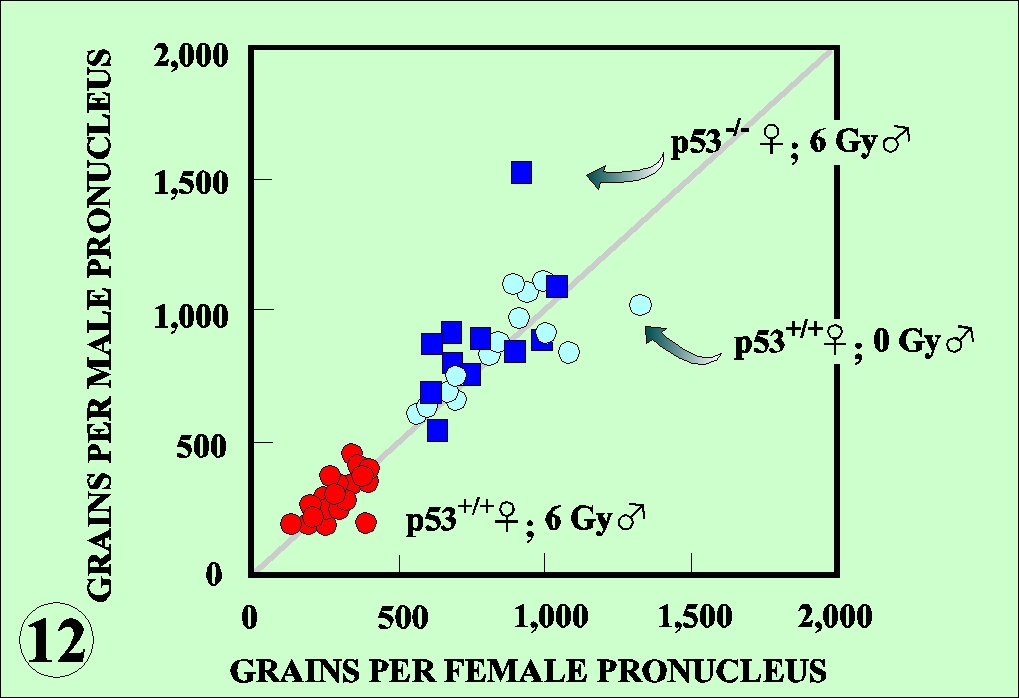
experimental condition, the activation of p53 was associated with the reduction of DNA replication rate
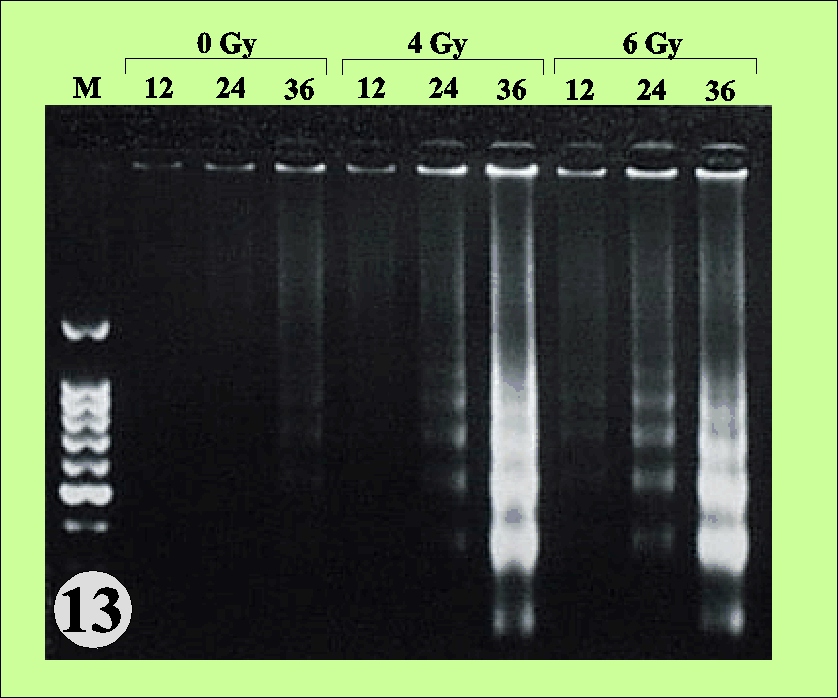
as measured by the incorporation of 3H-thymidine. Both nuclei were equally affected (Fig. 12). The radiation damage brought in by sperm is recognized by the egg sensing system and activates p53, which in turn regulates the DNA replication to facilitate the repair of the damage. The activation of p53 may also activate other p53 responsible genes. The X-irradiated Teratocarcinoma cells derived from mouse early embryonic cells display DNA fragmentation characteristic to the apoptosis (Fig. 13). It is thus higly likely that fertilized egg or early embryo with unrepaired damage may be eliminated by apoptotic killing and prevent the heritage of damaged genome to the next generation.
Sperm and egg are carriers of the genome to the next generation. In terms of the damage clearance system, mature sperm constitutes a particular stage in the life cycle because they lack DNA repair activity and therefore oxidative DNA damage due to energy metabolism and other damage inflicted by environmental agents including radiation can not be repaired. The living organisms must have evolved an excellent system to avoid such detrimental effects introduced by sperm. In the experimental system with the mouse eggs, it was found that p53 was playing a key role in the surveillance of the carried-in damage (Fig. 10). The p53 responsible LacZ contruct was injected into
female pronucleus of the egg fertilized by X-irradiated sperm, and the activation of p53 was studied by
the expression of nuclear localizing LacZ (-galactosidase) on X-gal base. Only when the p53+/+ eggs
were fertilized with X-irradiated sperm, both male and female pronuclei were stained, indicating that the
activation of p53 and translocation of the expressed -galactosidase into nuclei (Fig. 11). In this
experimental condition, the activation of p53 was associated with the reduction of DNA replication rate
as measured by the incorporation of 3H-thymidine. Both nuclei were equally affected (Fig. 12). The radiation damage brought in by sperm is recognized by the egg sensing system and activates p53, which in turn regulates the DNA replication to facilitate the repair of the damage. The activation of p53 may also activate other p53 responsible genes. The X-irradiated Teratocarcinoma cells derived from mouse early embryonic cells display DNA fragmentation characteristic to the apoptosis (Fig. 13). It is thus higly likely that fertilized egg or early embryo with unrepaired damage may be eliminated by apoptotic killing and prevent the heritage of damaged genome to the next generation.
5. Bioregulation of Radiation Effects in The Space Environment
 The biological effects of radiation are not simply the consequences of physico-chemical interaction of radiations with target molecules but are the integrated consequences of the regulatory mechanisms that have been acquired during the evolution of living organisms. Apart from the heavy charged particles (HZE) and neutrons, microgravity is the environment that the living organisms on earth have never experienced during their evolution. Therefore, the biological effect of radiation in space is entirely new problem (Fig. 14). Our previous experiment with Drosophila in Space Shuttle (Endeavor) showed a significant increase in recessive lethal mutation in the flight groups as compared with ground controls. (Biol. Sci. Space, 11:346, 1997) (Fig.15).
The biological effects of radiation are not simply the consequences of physico-chemical interaction of radiations with target molecules but are the integrated consequences of the regulatory mechanisms that have been acquired during the evolution of living organisms. Apart from the heavy charged particles (HZE) and neutrons, microgravity is the environment that the living organisms on earth have never experienced during their evolution. Therefore, the biological effect of radiation in space is entirely new problem (Fig. 14). Our previous experiment with Drosophila in Space Shuttle (Endeavor) showed a significant increase in recessive lethal mutation in the flight groups as compared with ground controls. (Biol. Sci. Space, 11:346, 1997) (Fig.15).
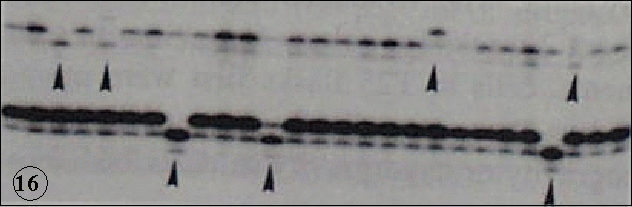
HZE were confirmed to show high relative biological effectiveness (RBE) in the induction of mutation s and malignant transformation (Int. J. Radiat. Biol., 74:239, 1998; J. Radiat. Res., 39:193, 1998; Adv. Space Res., 1999, in press). However, the amount of radiations during flight can not simply explain the increase of mutations. The biological response to microgravity is assumed to play a significant role in the amplification of mutations. To make an insight into this problem genetic stability has been tested with cultured human tumor cells in the simulated microgravity produced by clinostat. The results showed the increase in the mutation of microsatellite loci, indicating the induction of genetic instability (Mut. Res., 426:1, 1999) (Fig. 16). It should be also noted that hypergravity induces the accumulation of p53 and p21 gene products, which are the key proteins in the recognition and precessing of DNA damage and cell cycle regulation. With all these lines of experiments together, the cellular stress response afforded by the gravity changes are suggested to modulate the biological effects of radiations. Radiation effects in the microgaravity may not only provide an understanding of risk of radiation in space but also provide a better understanding of bioreguration of radiation effects on earth.
s and malignant transformation (Int. J. Radiat. Biol., 74:239, 1998; J. Radiat. Res., 39:193, 1998; Adv. Space Res., 1999, in press). However, the amount of radiations during flight can not simply explain the increase of mutations. The biological response to microgravity is assumed to play a significant role in the amplification of mutations. To make an insight into this problem genetic stability has been tested with cultured human tumor cells in the simulated microgravity produced by clinostat. The results showed the increase in the mutation of microsatellite loci, indicating the induction of genetic instability (Mut. Res., 426:1, 1999) (Fig. 16). It should be also noted that hypergravity induces the accumulation of p53 and p21 gene products, which are the key proteins in the recognition and precessing of DNA damage and cell cycle regulation. With all these lines of experiments together, the cellular stress response afforded by the gravity changes are suggested to modulate the biological effects of radiations. Radiation effects in the microgaravity may not only provide an understanding of risk of radiation in space but also provide a better understanding of bioreguration of radiation effects on earth.
 Radiation Biology Center, founded in 1976, is a national cooperative research institute for the fundamental research on the effects of radiations. Toward the 21st century, major concerns in radiation biology have shifted to the biological effects of low dose and low dose-rate radiations, where there is an increasing need for the dissection based on the fundamental life science. In 1995, the Center was appointed as a Center of Excellence (COE). In this context, the Center started a new research project "Bioregulation of Radiation Response" as a COE program, which covered the areas relating with (1) gene regulation of radiation response, (2) molecular mechanisms of DNA repair, (3) gene regulation of cell cycle, (4) mechanisms of genome stability, and (5) higher ordered regulation. The emphasis has been placed on the fusion of radiation effects and life science as recommended by the External Review Committee, which met in 1995 and 1996. This brochure summarizes some innovative topics in the progress of the COE program performed in 1995-1999.
Radiation Biology Center, founded in 1976, is a national cooperative research institute for the fundamental research on the effects of radiations. Toward the 21st century, major concerns in radiation biology have shifted to the biological effects of low dose and low dose-rate radiations, where there is an increasing need for the dissection based on the fundamental life science. In 1995, the Center was appointed as a Center of Excellence (COE). In this context, the Center started a new research project "Bioregulation of Radiation Response" as a COE program, which covered the areas relating with (1) gene regulation of radiation response, (2) molecular mechanisms of DNA repair, (3) gene regulation of cell cycle, (4) mechanisms of genome stability, and (5) higher ordered regulation. The emphasis has been placed on the fusion of radiation effects and life science as recommended by the External Review Committee, which met in 1995 and 1996. This brochure summarizes some innovative topics in the progress of the COE program performed in 1995-1999. Photoreactivation is the most efficient system to repair UV-induced DNA damage in that it converts light energy to electron, which in turn directly splits the dimerized bases into original conformation. Unlike cyclobutane pyrimidine dimer (CDP), (6-4) photoproduct has long been believed not to be a substrate of photorepair because of its specific steric structure. However, we identified a protein that specifically binds to (6-4) photoproduct, and finally cloned a gene (6-4) photolyase, Dm64, from Drosophila (Science, 272:109, 1996). Surprisingly, while its high sequence homology to CPD photolyase, the Dm64 also showed a high homology to blue-light photoreceptor (cryptochrome, CRY) in plant. Both have FAD chromophore, which catalyze photorepair in the case of photolyase or transduce photosignal in the case of CRY by transferring electron as a result of energy transfer from a second light-harvesting chromophore, MTHF or 8-HDF (Nucleic Acid Res., 25:764, 1997; J. Biol. Chem., 272:32591, 1997) (Fig. 1). We further cloned genes with high homology to Dm64 from human and Drosophila cells, herein called HsCRY and DCRY, which did not catalyze the photorepair but shared characteristics of CRY(Science, 272:764, 1996; Mut. Res., 384:195, 1997; Genes to Cells, 4:57, 1999). Thus, photolyases and CRY form a structurally and functionally related families which evolved by gene duplication ( J. Mol. Evol., 45:535, 1997)。Furthermore, the CRY was demonstrated to play a role in circadian rhythm (Genes to Cells, 4:57, 1999). Other recent studies indicate that CRY has a pivotal role in the circadian rhythm coupled with other regulator molecules such as TIM/PER and CLOCK/BMAL (Fig. 2). The April issue of Science in this year focused on a molecular diversity of photo-sensing in the repair and circadian rhythm (Fig.3).
Photoreactivation is the most efficient system to repair UV-induced DNA damage in that it converts light energy to electron, which in turn directly splits the dimerized bases into original conformation. Unlike cyclobutane pyrimidine dimer (CDP), (6-4) photoproduct has long been believed not to be a substrate of photorepair because of its specific steric structure. However, we identified a protein that specifically binds to (6-4) photoproduct, and finally cloned a gene (6-4) photolyase, Dm64, from Drosophila (Science, 272:109, 1996). Surprisingly, while its high sequence homology to CPD photolyase, the Dm64 also showed a high homology to blue-light photoreceptor (cryptochrome, CRY) in plant. Both have FAD chromophore, which catalyze photorepair in the case of photolyase or transduce photosignal in the case of CRY by transferring electron as a result of energy transfer from a second light-harvesting chromophore, MTHF or 8-HDF (Nucleic Acid Res., 25:764, 1997; J. Biol. Chem., 272:32591, 1997) (Fig. 1). We further cloned genes with high homology to Dm64 from human and Drosophila cells, herein called HsCRY and DCRY, which did not catalyze the photorepair but shared characteristics of CRY(Science, 272:764, 1996; Mut. Res., 384:195, 1997; Genes to Cells, 4:57, 1999). Thus, photolyases and CRY form a structurally and functionally related families which evolved by gene duplication ( J. Mol. Evol., 45:535, 1997)。Furthermore, the CRY was demonstrated to play a role in circadian rhythm (Genes to Cells, 4:57, 1999). Other recent studies indicate that CRY has a pivotal role in the circadian rhythm coupled with other regulator molecules such as TIM/PER and CLOCK/BMAL (Fig. 2). The April issue of Science in this year focused on a molecular diversity of photo-sensing in the repair and circadian rhythm (Fig.3).

 The radioadaptive response is an acquirement of radioresistance of the cells previously irradiated with low dose radiations. Its molecular mechanisms are largely unknown. In cultured mouse cells, adaptive response has a dose limitation up to 100 mSv, continues for more than 20 hours, and renders the cells resistant to mutation induction and cell killing, but not for malignant transformation, by subsequent radiations (Int. J. Radiat. Biol., 68:281, 1995). The kinetics led us to propose a new interpretation for the inverse dose-rate effect of low dose-rate exposures (Mut. Res., 358:207, 1996). The dose recognition and sustained nature of the adaptive response were found to be regulated by a nexus of PKC-p38MAPK-PLC1 feedback signal transduction pathway (Exp. Cell Res., 1999, in press). The activation of PKC continues for long time after low dose irradiation while it is immediately down-regulated after high dose. The activation of PKC is also coordinated with the adaptive response and activation of p38MAPK, which is physically associated with PLC1. Inhibitor of either one of the kinases inhibits activation of another and abrogates adaptive response. The adaptive response is dependent on p53, but not on the ATM or SCID facor (Fig. 5), and furthermore it is associated with the reduction of spontaneous and radiation-induced apoptosis. The in vitro assay for the rejoining of DNA double strand breaks (dsb) indicates that the adaptive response enhances the efficiency and fidelity of the rejoining of DNA dsb (Fig. 6). These lines of evidence suggest that the adaptive response is a reflection of the inducible efficient repair of DNA dsb which is otherwise death signal and substrate for mutation, chromosome aberration and reproductive cell death. Thus, the living organisms seem to have evolved an efficient system to escape from the detrimental effects on their genome.
The radioadaptive response is an acquirement of radioresistance of the cells previously irradiated with low dose radiations. Its molecular mechanisms are largely unknown. In cultured mouse cells, adaptive response has a dose limitation up to 100 mSv, continues for more than 20 hours, and renders the cells resistant to mutation induction and cell killing, but not for malignant transformation, by subsequent radiations (Int. J. Radiat. Biol., 68:281, 1995). The kinetics led us to propose a new interpretation for the inverse dose-rate effect of low dose-rate exposures (Mut. Res., 358:207, 1996). The dose recognition and sustained nature of the adaptive response were found to be regulated by a nexus of PKC-p38MAPK-PLC1 feedback signal transduction pathway (Exp. Cell Res., 1999, in press). The activation of PKC continues for long time after low dose irradiation while it is immediately down-regulated after high dose. The activation of PKC is also coordinated with the adaptive response and activation of p38MAPK, which is physically associated with PLC1. Inhibitor of either one of the kinases inhibits activation of another and abrogates adaptive response. The adaptive response is dependent on p53, but not on the ATM or SCID facor (Fig. 5), and furthermore it is associated with the reduction of spontaneous and radiation-induced apoptosis. The in vitro assay for the rejoining of DNA double strand breaks (dsb) indicates that the adaptive response enhances the efficiency and fidelity of the rejoining of DNA dsb (Fig. 6). These lines of evidence suggest that the adaptive response is a reflection of the inducible efficient repair of DNA dsb which is otherwise death signal and substrate for mutation, chromosome aberration and reproductive cell death. Thus, the living organisms seem to have evolved an efficient system to escape from the detrimental effects on their genome. 

 DNA double strand breaks (dsb) are the integral components of the biological effects of radiations. In bacterial system, DNA recombination has been implicated for the repair of dsb. Recent cloning of recombination- related mammalian genes and their disruption in chicken B cells made it possible to analyze recombinational events in radiation effects in higher organisms. DNA dsb may be repaired by two processes; non-homologous end joining (NHEJ) or homologous recombination (HR) (Fig. 7). The disruption of linear order of DNA is manifested as chromosome structural aberrations (Fig. 8). The RAD51-deficient chicken cells accumulate iso-chromatid breaks, indicating that RAD51 plays
an essential role in repairing spontaneously occurring chromosome breaks in DNA replications (EMBO
J., 17:598, 1998). The analysis in KU70/RAD54 double knock-out cells indicates that NHEJ plays a
major role in DNA dsb in G1 phase, which is supplemented by HR pathways in association with DNA replication (EMBO J., 17:5497, 1998). Sister chromatid exchange (SCE) is a switch of a newly synthesized chromatid with its sister. It was evident that KU70 or NHEJ was not involved in SCE formation. However, the spontaneous and
chemical (mitomycin)-induced SCE was suppressed to occur by the disruption of HR-related genes
(Mol. Cell. Biol., 19:5166, 1999). Surprisingly, chromatid aberrations (CA) were also suppressed to
occur in the cells deficient of HR-related genes except for XRCC2/3 and RAD51D genes (Fig. 9). The observations provide the first genetic evidence that SCE and chemical-induced CA are mediated by HR pathway, and moreover show that HR gene families are cooperatively involved in the recombinational repair while each has a distinct role (Fig. 9).
DNA double strand breaks (dsb) are the integral components of the biological effects of radiations. In bacterial system, DNA recombination has been implicated for the repair of dsb. Recent cloning of recombination- related mammalian genes and their disruption in chicken B cells made it possible to analyze recombinational events in radiation effects in higher organisms. DNA dsb may be repaired by two processes; non-homologous end joining (NHEJ) or homologous recombination (HR) (Fig. 7). The disruption of linear order of DNA is manifested as chromosome structural aberrations (Fig. 8). The RAD51-deficient chicken cells accumulate iso-chromatid breaks, indicating that RAD51 plays
an essential role in repairing spontaneously occurring chromosome breaks in DNA replications (EMBO
J., 17:598, 1998). The analysis in KU70/RAD54 double knock-out cells indicates that NHEJ plays a
major role in DNA dsb in G1 phase, which is supplemented by HR pathways in association with DNA replication (EMBO J., 17:5497, 1998). Sister chromatid exchange (SCE) is a switch of a newly synthesized chromatid with its sister. It was evident that KU70 or NHEJ was not involved in SCE formation. However, the spontaneous and
chemical (mitomycin)-induced SCE was suppressed to occur by the disruption of HR-related genes
(Mol. Cell. Biol., 19:5166, 1999). Surprisingly, chromatid aberrations (CA) were also suppressed to
occur in the cells deficient of HR-related genes except for XRCC2/3 and RAD51D genes (Fig. 9). The observations provide the first genetic evidence that SCE and chemical-induced CA are mediated by HR pathway, and moreover show that HR gene families are cooperatively involved in the recombinational repair while each has a distinct role (Fig. 9).

 Sperm and egg are carriers of the genome to the next generation. In terms of the damage clearance system, mature sperm constitutes a particular stage in the life cycle because they lack DNA repair activity and therefore oxidative DNA damage due to energy metabolism and other damage inflicted by environmental agents including radiation can not be repaired. The living organisms must have evolved an excellent system to avoid such detrimental effects introduced by sperm. In the experimental system with the mouse eggs, it was found that p53 was playing a key role in the surveillance of the carried-in damage (Fig. 10). The p53 responsible LacZ contruct was injected into
female pronucleus of the egg fertilized by X-irradiated sperm, and the activation of p53 was studied by
the expression of nuclear localizing LacZ (-galactosidase) on X-gal base. Only when the p53+/+ eggs
were fertilized with X-irradiated sperm, both male and female pronuclei were stained, indicating that the
activation of p53 and translocation of the expressed -galactosidase into nuclei (Fig. 11). In this
experimental condition, the activation of p53 was associated with the reduction of DNA replication rate
as measured by the incorporation of 3H-thymidine. Both nuclei were equally affected (Fig. 12). The radiation damage brought in by sperm is recognized by the egg sensing system and activates p53, which in turn regulates the DNA replication to facilitate the repair of the damage. The activation of p53 may also activate other p53 responsible genes. The X-irradiated Teratocarcinoma cells derived from mouse early embryonic cells display DNA fragmentation characteristic to the apoptosis (Fig. 13). It is thus higly likely that fertilized egg or early embryo with unrepaired damage may be eliminated by apoptotic killing and prevent the heritage of damaged genome to the next generation.
Sperm and egg are carriers of the genome to the next generation. In terms of the damage clearance system, mature sperm constitutes a particular stage in the life cycle because they lack DNA repair activity and therefore oxidative DNA damage due to energy metabolism and other damage inflicted by environmental agents including radiation can not be repaired. The living organisms must have evolved an excellent system to avoid such detrimental effects introduced by sperm. In the experimental system with the mouse eggs, it was found that p53 was playing a key role in the surveillance of the carried-in damage (Fig. 10). The p53 responsible LacZ contruct was injected into
female pronucleus of the egg fertilized by X-irradiated sperm, and the activation of p53 was studied by
the expression of nuclear localizing LacZ (-galactosidase) on X-gal base. Only when the p53+/+ eggs
were fertilized with X-irradiated sperm, both male and female pronuclei were stained, indicating that the
activation of p53 and translocation of the expressed -galactosidase into nuclei (Fig. 11). In this
experimental condition, the activation of p53 was associated with the reduction of DNA replication rate
as measured by the incorporation of 3H-thymidine. Both nuclei were equally affected (Fig. 12). The radiation damage brought in by sperm is recognized by the egg sensing system and activates p53, which in turn regulates the DNA replication to facilitate the repair of the damage. The activation of p53 may also activate other p53 responsible genes. The X-irradiated Teratocarcinoma cells derived from mouse early embryonic cells display DNA fragmentation characteristic to the apoptosis (Fig. 13). It is thus higly likely that fertilized egg or early embryo with unrepaired damage may be eliminated by apoptotic killing and prevent the heritage of damaged genome to the next generation.
 The biological effects of radiation are not simply the consequences of physico-chemical interaction of radiations with target molecules but are the integrated consequences of the regulatory mechanisms that have been acquired during the evolution of living organisms. Apart from the heavy charged particles (HZE) and neutrons, microgravity is the environment that the living organisms on earth have never experienced during their evolution. Therefore, the biological effect of radiation in space is entirely new problem (Fig. 14). Our previous experiment with Drosophila in Space Shuttle (Endeavor) showed a significant increase in recessive lethal mutation in the flight groups as compared with ground controls. (Biol. Sci. Space, 11:346, 1997) (Fig.15).
The biological effects of radiation are not simply the consequences of physico-chemical interaction of radiations with target molecules but are the integrated consequences of the regulatory mechanisms that have been acquired during the evolution of living organisms. Apart from the heavy charged particles (HZE) and neutrons, microgravity is the environment that the living organisms on earth have never experienced during their evolution. Therefore, the biological effect of radiation in space is entirely new problem (Fig. 14). Our previous experiment with Drosophila in Space Shuttle (Endeavor) showed a significant increase in recessive lethal mutation in the flight groups as compared with ground controls. (Biol. Sci. Space, 11:346, 1997) (Fig.15).
 s and malignant transformation (Int. J. Radiat. Biol., 74:239, 1998; J. Radiat. Res., 39:193, 1998; Adv. Space Res., 1999, in press). However, the amount of radiations during flight can not simply explain the increase of mutations. The biological response to microgravity is assumed to play a significant role in the amplification of mutations. To make an insight into this problem genetic stability has been tested with cultured human tumor cells in the simulated microgravity produced by clinostat. The results showed the increase in the mutation of microsatellite loci, indicating the induction of genetic instability (Mut. Res., 426:1, 1999) (Fig. 16). It should be also noted that hypergravity induces the accumulation of p53 and p21 gene products, which are the key proteins in the recognition and precessing of DNA damage and cell cycle regulation. With all these lines of experiments together, the cellular stress response afforded by the gravity changes are suggested to modulate the biological effects of radiations. Radiation effects in the microgaravity may not only provide an understanding of risk of radiation in space but also provide a better understanding of bioreguration of radiation effects on earth.
s and malignant transformation (Int. J. Radiat. Biol., 74:239, 1998; J. Radiat. Res., 39:193, 1998; Adv. Space Res., 1999, in press). However, the amount of radiations during flight can not simply explain the increase of mutations. The biological response to microgravity is assumed to play a significant role in the amplification of mutations. To make an insight into this problem genetic stability has been tested with cultured human tumor cells in the simulated microgravity produced by clinostat. The results showed the increase in the mutation of microsatellite loci, indicating the induction of genetic instability (Mut. Res., 426:1, 1999) (Fig. 16). It should be also noted that hypergravity induces the accumulation of p53 and p21 gene products, which are the key proteins in the recognition and precessing of DNA damage and cell cycle regulation. With all these lines of experiments together, the cellular stress response afforded by the gravity changes are suggested to modulate the biological effects of radiations. Radiation effects in the microgaravity may not only provide an understanding of risk of radiation in space but also provide a better understanding of bioreguration of radiation effects on earth.